Translate this page into:
Implementation of a Dual-Column Liquid Chromatography-Tandem Mass-Spectrometry Method for the Quantification of Isavuconazole in Clinical Practice
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
Therapeutic drug monitoring (TDM) of isavuconazole, which is a novel broad-spectrum antimycoticum against invasive fungal infections, ensures an effective exposure of the drug and minimizes the risk of toxicity. This study is aimed at evaluating the analytical performance of a dual-column liquid chromatography-tandem mass-spectrometry (LC-MS/MS) method for isavuconazole quantification.
Materials and Methods
The method was performed on a Voyager TSQ Quantum triple quadrupole instrument equipped with an Ultimate 3000 chromatography system (Thermo Fisher Scientific, San Jose, California, United States). Analytical and preanalytical requirements of the isavuconazole LC-MS/MS method were evaluated. Sample stability measurements were performed at room temperature (RT) and in serum tubes with separator gel.
Results
The isavuconazole LC-MS/MS method was linear over the concentration range of 0.2 to 12.8 mg/L. The coefficient of determination (r2) always exceeded 0.999. Within- and between-run precision ranged between 1.4 to 2.9% and 1.5 to 3.0%, the recovery between 93.9 and 102.7%. At RT, serum samples were stable for 3 days. Isavuconazole serum concentrations were significantly lower after incubation (18 hours) in serum tubes with separator gel at RT.
Conclusion
The dual-column isavuconazole LC-MS/MS is a reliable tool for the TDM of isavuconazole. Serum samples are stable for at least 3 days and should be collected in tubes without separator gel.
Keywords
antifungal agents
isavuconazole
liquid chromatographytandem mass-spectrometry
therapeutic drug monitoring
triazole
Introduction
Fungal infections are a high-risk factor of morbidity and mortality in patients with immunosuppressive or cancer chemotherapy. Triazole antifungal agents (e.g., voriconazole, itraconazole, posaconazole, isavuconazole) are important drugs for the prophylaxis and treatment of invasive fungal diseases (IFDs). Isavuconazole is a new generation, broad-spectrum triazole, which is commonly used for IFD caused by a range of rare or multiple fungal species.[1,2] This agent inhibits the 14α-demethylase, which affects the ergosterol biosynthesis and disrupts the fungal cell membrane structure and function.[3]
Serum concentrations of triazoles sometimes require therapeutic drug monitoring (TDM). Since voriconazole, itraconazole, and posaconazole show a high level of intra- and interindividual variability and a narrow therapeutic window, antifungal TDM is generally indicated for these agents.[4,5] In a previous study isavuconazole was shown to have a high bioavailability and dose-proportional pharmacokinetic, which demonstrated no relevant differences between patients with IFD and healthy subjects.[6]
Several methods have been established for the quantification of serum concentrations of antifungal agents, including liquid chromatography-tandem mass-spectrometry (LC-MS/MS), high-performance liquid chromatography (HPLC), and the bioassay.[4] LC-MS/MS is a powerful and valuable tool with the advantages of high specificity, precision, and the potential of simultaneous separation of multiple analytes.[7,8] However, this method is not widely available compared with the HPLC technology and the cheap and simple bioassay.[4]
To date, no Food and Drug Administration (FDA)-approved assay for the quantification of isavuconazole serum concentrations is available.[9] Therefore, a thorough evaluation of the analytical performance by the use of isavuconazole LC-MS/MS instrumentation is recommended by clinical laboratories before this method can be used in clinical routine.
The present study aimed at evaluating a dual-column LC-MS/MS method for the quantification of isavuconazole in serum. Evaluation consisted of the assessment of the linearity, precision, recovery, limit of quantification (LOQ), and limit of detection (LOD). Additionally, we performed sample stability measurements at room temperature (RT) in serum tubes with separator gel.
Materials and Methods
Instrumentation and Conditions
The method was performed on a Voyager TSQ Quantum triple quadrupole instrument equipped with an Ultimate 3000 chromatography system (Thermo Fisher Scientific, San Jose, California, United States). Chromeleon Xpress software for device management and LCQuanTM 2.7 for data processing were used. Isavuconazole and its deuterated isotope were kindly provided by Basilea (Basel, Switzerland). Calibrators and controls were prepared by spiking drug free serum from healthy donors with stock solutions. In brief, 20-µL serum samples (calibrators or controls) were deproteinized by adding 100-µL ice-cold methanol containing the internal standard (IS) (isavuconazole-d4, 1 mg/mL). After vortexing and centrifugation at 24,000 × g (5 minutes), the clear supernatant was tenfold diluted with 5% ACN. Ten microliter were loaded on a trapping column (POROS R1 20, 2.1 × 30 mm, Thermo Fisher Scientific) with mobile phase 1 (5:95 v/v ACN/water). After a short washing period the analytes were transferred and separated on a Luna 5-μm Phenyl–Hexyl column 100A 50 × 2.1 mm (Phenomenex, Aschaffenburg, Germany) with a linear gradient of mobile phase 2 (0.1% formic acid in MS grade water) and mobile phase 3 (0.1% formic acid in MS grade ACN). Isavuconazole and the IS were monitored in a positive multiple reaction monitoring mode using characteristic precursor–product ion transitions: m/z 438.1→214.9 (438.1→368.9 as a second qualifier) and m/z 442.1→218.9 (442.1→372.9 as a second qualifier), respectively.
Method Evaluation
A calibration curve was prepared using serum samples spiked with six different isavuconazole concentrations ranging from 0.2 to 12.8 (0.2, 0.8, 1.6, 3.2, 6.4, 12.8) mg/L and analyzed in triplicate.[10] The linear calibration curve was constructed by plotting the ratios of peak areas of the analyte divided by the corresponding IS against the concentration of each compound. The data from the standard curve were analyzed using regression analysis to obtain the slope, the intercept, and the correlation coefficient (r2). The acceptance criteria of the recalculated measured values of each calibration point were ≤ 15%.
The within-run precision was assessed by five replicate analyses of low, medium, and high isavuconazole concentrations of a serumpool on one day. The between-run precision was determined by replicate analyses of low, medium, and high isavuconazole concentrations of the same serumpool on 5 consecutive days.[11] The precision goal for each concentration was not to exceed 15% of the coefficient of variation (CV).[12]
The recovery of isavuconazole was determined three times at three different concentrations (0.5, 2.5, and 10 mg/L) by calculating the percentage difference between the area of analytes of authentic pure substance added to a patient pool before and after precipitation.
The LOD was defined as the lowest concentration, which produced a signal at least three times higher than the average background noise. The LOQ was defined as the lowest concentration possible to quantify imprecision of < 10%.[13,14]
This study was performed in accordance with the latest version of the declaration of Helsinki (Fortaleza 2013) and was approved by the Ethical Committee of the Medical University of Graz (Graz, Austria).
Preanalytical Analyses
Since specimens are usually handled and transported to the laboratory at RT, we performed isavuconazole stability measurements at constant 25°C RT in our laboratory. We stored a patient pool (n = 10) at RT with daylight exposure. For 3 days, one portion of the pool was frozen at -80°C on each day until batch analyses of all frozen samples were performed immediately after this period.
To detect if separator gel absorbs isavuconazole, we incubated the triplicate heparinized patient samples spiked with different concentrations of isavuconazole (1.7, 4.6, and 12.8 mg/L) for 18 hours in serum tubes with separator gel at RT and compared the measured concentrations after the incubation with the initial measurements before incubation.
Statistical Analysis
The paired t-test was calculated to assess the differences of mean isavuconazole concentrations at different serum levels (1.7, 4.6, and 12.8 mg/L) before and after incubation in serum tubes with separator gel. A p-value < 0.05 was considered statistically significant. The analyses were performed using SPSS 25.0 statistical software (SPSS Inc., Chicago, Illinois, United States).
Results
Analytical Performance
The chromatographic separation of isavuconazole with the LC-MS/MS method in human serum is illustrated in ►Figs. 1A and B. The calibration curve was linear over the range from 0.2 to 12.8 mg/L (►Fig. 2). The evaluation results of the six-point calibration curve measurements are shown in ►Table 1. The coefficient of determination (r2) always exceeded 0.999.
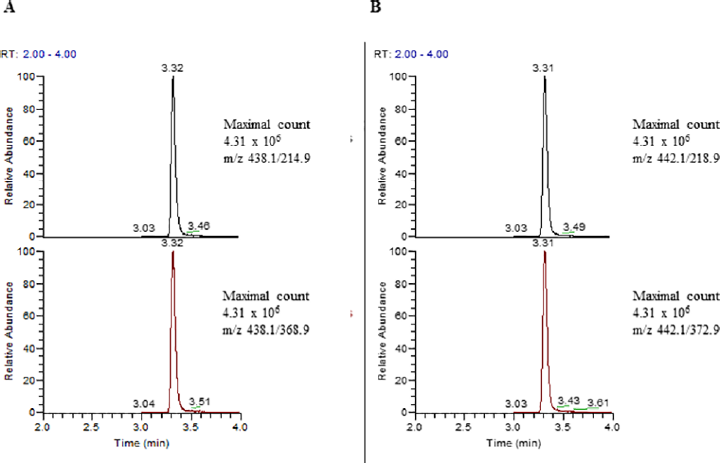
- Representative chromatogram of a patient with 3.63 mg/L isavuconazole. Every peak has over 20 measuring points. Peaks are shown without smoothing. (A) Isavuconazole: m/z 438.1→214.9 as the quantifier and 438.1→368.9 as a second qualifier; (B) Internal standard isavuconazole d4: m/z 442.1→218.9 as the quantifier and 442.1→372.9 as a second qualifier.
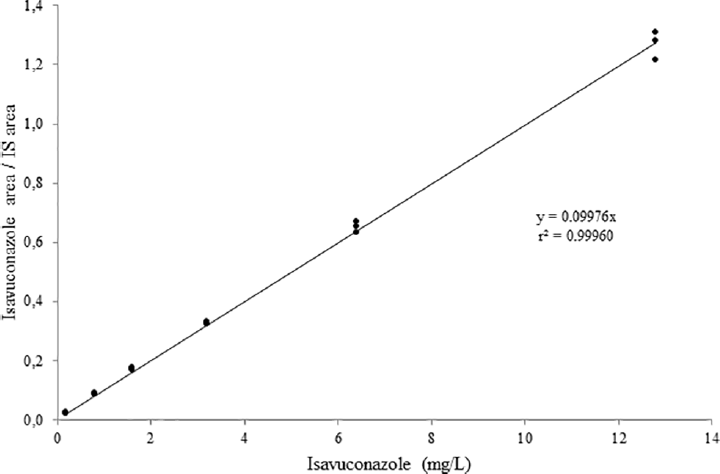
- Calibration curve of isavuconazole: y = 0.0997x, r2 = 0.999. IS, internal standard.
| Isavuconazole (mg/L) | Mean | SD | CV (%) | MaxD (%) |
|---|---|---|---|---|
| Calibrator 1 (0.2) | 0.21 | 0.002 | 1.00 | 8.0 |
| Calibrator 2 (0.8) | 0.85 | 0.020 | 2.29 | 9.3 |
| Calibrator 3 (1.6) | 1.69 | 0.045 | 2.64 | 8.4 |
| Calibrator 4 (3.2) | 3.21 | 0.027 | 0.84 | 1.2 |
| Calibrator 5 (6.4) | 6.42 | 0.192 | 3.0 | –2.9 |
| Calibrator 6 (12.8) | 12.51 | 0.463 | 3.7 | –6.3 |
Abbreviations: CV, coefficient of variation; MaxD, maximum deviation of the recalculated single values of the determination in triplicate; SD, standard deviation.
The characteristics of the analytical method are presented in ►Table 2. The within-run CVs varied between 1.4 and 2.9% and the between-run CVs ranged between 1.5 and 3.0%. The recovery result was highly satisfactory. The LOQ and LOD were below the lowest measured values. On the LOQ (0.1 µg/mL) the CV was < 10%.
| Isavuconazole | |||
|---|---|---|---|
| Working range (mg/L) | 0.2–12.8 | ||
| Calibration curve | |||
| Slope | 0.0997 | ||
| Intercept | 0 | ||
| Correlation r2 | 0.999 | ||
| Within-run precision (n = 5) | Low | Medium | High |
| Mean (mg/L) | 2.85 | 4.78 | 6.62 |
| SD (mg/L) | 0.09 | 0.07 | 0.12 |
| CV (%) | 2.96 | 1.4 | 1.78 |
| Between-run precision (n = 5) | Low | Medium | High |
| Mean (mg/L) | 2.89 | 4.79 | 6.59 |
| SD (mg/L) | 0.09 | 0.09 | 0.1 |
| CV (%) | 3.01 | 1.81 | 1.53 |
| Recovery (range, %) | 93.9–102.7 | ||
| Limit of quantification | |||
| LOQ (mg/L) | 0.1 | ||
| SD (mg/L) | 0.006 | ||
| CV (%) | 5.7 | ||
| Limit of detection | |||
| LOD (mg/L threefold SD of the baseline noise) | 0.04 | ||
| Stability measurements | |||
| Stability at RT | 3 d | ||
Abbreviations: CV, coefficient of variation; LOQ, limit of quantification; LOD, limit of detection; RT, room temperature; SD, standard deviation.
Preanalytical Measurements
As shown in ►Fig. 3, isavuconazole serum samples were stable for at least 3 days at RT. The mean (± standard deviation) serum isavuconazole concentration of all measured time points (n = 4) was 3.63 (± 0.13), respectively.
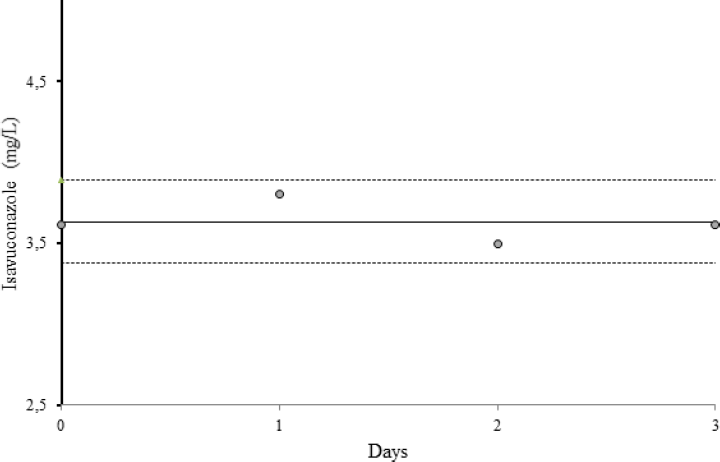
- Isavuconazole stability measurements of a patient pool (n = 10) at room temperature for 3 days. The solid line shows the mean isavuconazole concentration of all measurements (3.63 ± 0.13 mg/L), the dotted lines represent the twofold standard deviation (± 2 SD).
Isavuconazole concentrations were determined at different serum levels before and after incubation (18 hours) in serum tubes with separator gel at RT. The measured mean concentrations (± standard deviation) at the low (1.7 mg/L), medium (4.6 mg/L), and high (12.8 mg/L) serum level were significantly lower after incubation (1.60 ± 0.03, 4.23 ± 0.10, and 11.12 ± 0.29 mg/L) compared with measurements before (1.75 ± 0.01, 4.62 ± 0.02, 12.58 ± 0.28 mg/L) (p-values: 0.001, 0.003, and 0.003) incubation in blood collection tubes with separator gel, respectively.
Discussion
Herein, we described the development and evaluation of a dual-column LC-MS/MS method for the measurement of the new triazole isavuconazole in human serum. The method was linear within the range of 0.2 to 12.8 mg/L, which comprises the clinically expected isavuconazole through blood levels (median: 2.93 mg/L; range: 0.81–9.95 mg/L).[15,16] We prepared the calibration curve with six different isavuconazole concentrations in triplicate and fulfilled the recommended criteria, which propose the determination of five to seven calibrators at least tested in duplicate at each level.[10,17] The within- and between-run imprecisions varied between 1.4 and 3.0%. These results are in line with the LC-MS/MS acceptance criteria of ≤15% in the guidelines of biological method validation of the European Medicines Agency and the FDA.[12,17]
Here, we used the LC-MS/MS method for isavuconazole quantification. This technique was shown to be more capable of determining mixtures of drugs compared with the HPLC.[18] The HPLC technique is more time-consuming, has a lower throughput, and different substances may interfere with the analyte determination. Since quantification of isavuconazole serum concentrations is quite often recommended for critically ill patients with multidrug therapy, the LC-MS/MS is considered the method of choice in this patient setting.[19]
The selection of adequate LC columns is required for the development of an optimal quantitative LC-MS/MS method.[20] In the present study, we implemented a dual-column LC-MS/MS. The serial combination of two different columns is known to be more effective in view of separating drugs and metabolites in complex matrices compared with single column methods.[21-23]
To obtain valid results for TDM of isavuconazole, preanalytical influencing factors such as blood collection tubes, handling procedures, and storage conditions must be considered. Here, we performed stability measurements at RT, which is known the worst encumbering condition for specimens of blood. At ambient RT, which was constant at 25°C and monitored by continuous record of air conditioner in our laboratory, isavuconazole was observed to be highly stable for 3 days. In comparison, a recently published study reported good results of stability experiments at RT, which were performed for up to 6 hours only.[24] The same authors observed isavuconazole measurements to be stable at –20°C and –80°C for at least 5 weeks.[24] Another study found unextracted isavuconazole to be stable for 15 days at RT and 4°C.[25]
In this work, serum isavuconazole concentrations were significantly lower after incubation in blood collection tubes with separator gel at RT. The absorption of therapeutic drugs by barrier gel has been known as a potential reason of clinically important error in drug measurements for a long time.[26-29] Nevertheless, in the current literature, we could not find studies investigating separator gel effects on serum isavuconazole concentrations. A recently published study, which evaluated the impact of serum separator blood collection tubes on the stability of a panel of 167 drugs, demonstrated, that tubes without separator gel cause less drug interferences.[30] Therefore, these tubes are recommended for the quantification of drugs.[30]
The major limitation of this study is that individual serum concentrations of isavuconazole were not assessed in a patient setting.
Conclusion
The evaluated dual-column isavuconazole LC-MS/MS method described here shows a broad analytical range and meets the imprecision acceptance criteria of ≤ 15%. These data are indicative for a precise and reliable diagnostic tool for the TDM of isavuconazole in daily clinical routine. Isavuconazole serum samples should be collected in tubes without separator gel and are stable at RT for at least 3 days.
Compliance with Ethical Standards
This study was performed in accordance with the latest version of the declaration of Helsinki (Fortaleza 2013) and was approved by the Ethical Committee of the Medical University of Graz (Graz, Austria).
Authors’ Contribution
D.E., S.Z., M.H., R.K., and A.M. designed the study. D.E. and A.M. collected, analyzed, and interpreted the data. D.E. wrote the first draft of the manuscript and A.M. supervised this study project. All listed authors revised the manuscript critically and approved the last version of this manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding
None.
References
- Isavuconazole for treatment of rare invasive fungal diseases. Mycoses. 2018;61(08):518-533.
- [CrossRef] [PubMed] [Google Scholar]
- Isavuconazole for treatment of invasive fungal diseases caused by more than one fungal species. Mycoses. 2018;61(07):485-497.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the pharmacokinetics and clinical utility of isavuconazole for treatment of invasive fungal infections. Expert Opin Drug Metab Toxicol. 2012;8(06):759-765.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother. 2014;69(05):1162-1176.
- [CrossRef] [PubMed] [Google Scholar]
- Experience with therapeutic drug monitoring of three antifungal agents using an LC-MS/MS method in routine clinical practice. Clin Biochem. 2019;70:14-17.
- [CrossRef] [PubMed] [Google Scholar]
- Population pharmacokinetics of isavuconazole from phase 1 and phase 3 (SECURE) trials in adults and target attainment in patients with invasive infections due to aspergillus and other filamentous fungi. Antimicrob Agents Chemother. 2016;60(09):5483-5491.
- [CrossRef] [PubMed] [Google Scholar]
- LC-MS/MS in the routine clinical laboratory: has its time come. ?Anal Bioanal Chem. 2014;406(09/10):2289-2301.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of a commercial liquid-chromatography high-resolution mass-spectrometry method for the determination of hepcidin-25. Biochem Med (Zagreb). 2019;29(02):020701.
- [CrossRef] [PubMed] [Google Scholar]
- Quantification of serum voriconazole, isavuconazole, and posaconazole by liquid chromatography tandem mass spectrometry (LC-MS/MS) Curr Protoc Toxicol. 2018;76(01):e47.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach; approved guideline. In: CSLI Document EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2003.
- [Google Scholar]
- Evaluation of Precision of Quantitative Measurement Procedures; Approved Guideline. In: CSLI Document EP05–A3 (3rd). Wayne, PA: Clinical and Laboratory Standards Institute; 2014.
- [Google Scholar]
- Bioanalytical Method Validation—Guidance for Industry. Rockeville, MA: Food and Drug Administration; 2018.
- [Google Scholar]
- Harmonized guidelines for single laboratory validation of methods of analysis. Pure Appl Chem. 2002;74:835-855.
- [CrossRef] [Google Scholar]
- Reference values for plasma concentrations of asymmetrical dimethylarginine (ADMA) and other arginine metabolites in men after validation of a chromatographic method. Clin Chim Acta. 2007;384(01/02):141-148.
- [CrossRef] [PubMed] [Google Scholar]
- Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387(10/020):760-769.
- [CrossRef] [PubMed] [Google Scholar]
- Validation of an isavuconazole high-performance liquid chromatography assay in plasma for routine therapeutic drug monitoring applications. Ther Drug Monit. 2018;40(04):503-506.
- [CrossRef] [PubMed] [Google Scholar]
- Guideline on Bioanalytical Method Validation London: European Medicines Agency; 2011.
- Comparative LC-MS and HPLC analyses of selected antiepileptics and beta-blocking drugs. Farmaco. 2000;55(02):136-145.
- [CrossRef] [PubMed] [Google Scholar]
- Reliable and easy-to-use liquid chromatography-tandem mass spectrometry method for simultaneous analysis of fluconazole, isavuconazole, itraconazole, hydroxy-itraconazole, posaconazole, and voriconazole in human plasma and serum. Ther Drug Monit. 2017;39(05):505-513.
- [CrossRef] [PubMed] [Google Scholar]
- Screening ionisation and chromatography conditions for quantitative LC/MS methods. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(29):3581-3588.
- [CrossRef] [PubMed] [Google Scholar]
- Serial coupling of reversed-phase and hydrophilic interaction liquid chromatography to broaden the elution window for the analysis of pharmaceutical compounds. J Chromatogr A. 2008;1208(01/02):90-94.
- [CrossRef] [PubMed] [Google Scholar]
- Serially coupling hydrophobic interaction and reversed-phase chromatography with simultaneous gradients provides greater coverage of the metabolome. Metabolomics. 2015;11(05):1465-1470.
- [CrossRef] [PubMed] [Google Scholar]
- Recent advances in liquid and gas chromatography methodology for extending coverage of the metabolome. Curr Opin Biotechnol. 2017;43:77-85.
- [CrossRef] [PubMed] [Google Scholar]
- Isotope dilution LC-orbitrap-HRMS with automated sample preparation for the simultaneous quantification of 11 antimycotics in human serum. J Pharm Biomed Anal. 2019;166:398-405.
- [CrossRef] [PubMed] [Google Scholar]
- Development and validation of a liquid chromatography-tandem mass spectrometry assay for the simultaneous quantitation of 5 azole antifungals and 1 active metabolite. Clin Chim Acta. 2017;474:8-13.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of serum separator blood collection tubes on drug concentrations. Ther Drug Monit. 1983;5(03):359-362.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of use of gel-barrier sampling tubes on determination of some antiepileptic drugs in serum. Clin Chem. 1984;30(03):465-466.
- [CrossRef] [PubMed] [Google Scholar]
- Absorption of therapeutic drugs by barrier gels in serum separator blood collection tubes. Volume- and time-dependent reduction in total and free drug concentrations. Am J Clin Pathol. 1994;101(04):456-461.
- [CrossRef] [PubMed] [Google Scholar]
- Suitability of collection tubes with separator gels for collecting and storing blood samples for therapeutic drug monitoring (TDM) Clin Chem Lab Med. 2000;38(04):313-320.
- [CrossRef] [PubMed] [Google Scholar]
- The right blood collection tube for therapeutic drug monitoring and toxicology screening procedures: standard tubes, gel or mechanical separator? Clin Chim Acta. 2019;488:196-201.
- [CrossRef] [PubMed] [Google Scholar]






