Translate this page into:
A Rapid Method for Determination of Serum Methotrexate Using Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry and Its Application in Therapeutic Drug Monitoring
Address for correspondence: Raghavendra Lingaiah, MBBS, MD, Department of Pathology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Raebareli Road, Lucknow 226014, Uttar Pradesh, India (e-mail: raghulingaiah@gmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
Methotrexate (MTX) has anticancer therapeutic potential with multiple doses-related adverse effects and toxicities. Immunoassays for therapeutic monitoring of serum MTX have their own limitations. Liquid chromatography–tandem mass spectrometry (LC-MS/MS) is considered as the reference method; however, commercially availability of them is limited. We aimed to adapt/develop an in-house LC-MS/MS method for therapeutic monitoring of serum MTX.
Materials and Methods
Serum protein precipitation was performed using acetonitrile–water containing 250 µM solution of aminoacetophenone as internal standard (IS). Chromatographic separation was achieved on a C18 column with mobile phase of 0.1% solution of formic acid (solvent A) and acetonitrile (solvent B) at a flow rate of 0.4 mL/min. MS was performed under positive ion mode with mass transition for MTX and IS as m/z 455.1→308.1 and 136.2→94.1, respectively. The method was validated by following Bioanalytical Method Validation Guidance for Industry, 2018 and applied on leukemia patients' samples on MTX therapy.
Results
The correlation coefficient of eight serially diluted calibration standards of 0.09 to 12.5 µM was >0.99 and had linearity with > 95% precision and accuracy at analytical quality control levels. The lower limit of MTX quantification achieved was 0.09 µM with good intensity and sharp peak as compared with blank sample. The total run time of the assay was 5 minutes. The serum MTX levels obtained by this method in leukemia patients exhibited clinical correlation and an excellent agreement with commercial immunoassay used in parallel.
Conclusion
We were able to develop a rapid, sensitive, and cost-effective LC-MS/MS method suitable for therapeutic drug monitoring of MTX in routine clinical diagnostic laboratories.
Keywords
methotrexate
therapeutic drug monitoring
cancer
liquid chromatography
tandem mass
spectrometry
Introduction
Methotrexate (MTX), chemically 2, 4-diamino-N10-methylpteroylglutamic acid, is a prototype folate antagonist widely used to treat various types of cancer including acute lymphoblastic leukemia (ALL), osteosarcoma, and lymphomas. It prevents division of cancer cells by inhibiting dihydrofolate reductase, a key metabolic enzyme involved in cell division and proliferation.[1,2] A high dose (>1 g/m2) of MTX in multiple doses and schedules is commonly used in the treatment of cancer.[3–5] However, only a few fractions of doses are transformed to 7-hydroxy-methotrexate and most of the dose remain as such in urine which results in life-threatening side effects such as renal toxicity,[6–8] hepatotoxicity,[9–11] neurotoxicity,[12–14] hematologic toxicity,[15,16] pulmonary toxicity,[17–19] and many more, while doses of oral MTX are variable because of the interindividual variability of gastrointestinal absorption of this drug. High-dose MTX-induced renal toxicity is prevented through various mechanisms including alkalinization of urine and carboxypeptidases. Bone marrow and gastrointestinal mucosa are particularly susceptible to the effects of MTX; moreover, renal toxicity is frequently observed with a high dose of MTX.[6,8] Therefore, it needs a sensitive method to precisely measure serum MTX levels for better management of patients with malignant tumors and avoid the drug treatment-related clinical toxicities.
Different analytical approaches have been described for the determination of MTX and its metabolites, including enzyme immunoassays and chromatographic methods.[20,21] Homogenous enzyme immunoassay for the quantitative determination of MTX in human serum or plasma on automated clinical chemistry analyzers is available and is widely used for monitoring the MTX levels. Although easier and automated, this method cannot accurately detect MTX levels in patients who are given glucarpidase, an exogenous enzyme approved by the U.S. Food and Drug Administration (US-FDA) as a high-dose MTX rescue therapy.[22,23] This glucarpidase specifically degrades MTX into inactive metabolite 4-[[2,4-diamino-6-(pyridinyl) methyl]-methylamino]-benzoic acid (DAMPA), which cross-reacts with the MTX antibody used in the immunoassays giving unexpected and potentially misleading results. The increased serum levels of DAMPA continue to circulate for at least 5 to 7 days giving falsely elevated values of MTX. This might falsely alarm the clinician which might lead to reconsidering the therapeutic decisions of using MTX.[24,25] Recently, chromatography-based several methods coupled with ultraviolet-visible detector, fluorescence detector, and mass spectrometry (MS) have been introduced to overcome these limitations of immunoassays. Among these methods, liquid chromatography (LC) coupled with tandem MS (LC-MS/MS) has emerged as a potential tool for rapid, sensitive, and cost-effective method for therapeutic drug monitoring of MTX.[26,27] However, there are very few commercially available kits for the LC-MS/MS-based assays and application note of these kits may not be available for all LC-MS/MS instruments for application in routine patient care for therapeutic drug monitoring of MTX in clinical laboratories particularly in India.
Therefore, the aim of this study was to develop ultra-high-performance LC-MS/MS (UHPLC-MS/MS) method for the determination of MTX concentration in human serum and validation of this method for therapeutic drug monitoring of MTX in ALL patients.
Materials and Methods
The assay development and validation were done on Vanquish UHPLC system (Thermo Fisher Scientific, United States) coupled with TSQ Quantis, a triple quadrupole mass spectrometer (Thermo Fisher Scientific).
Method Development
Chemical and Reagents
MTX reference standards, MTX for peak identification, and p-aminoacetophenone were purchased from Sigma-Aldrich (United States). High-performance LC (HPLC) grade methanol, acetonitrile, and formic acid were obtained from Merck Life Sciences (United States). Pooled and individual human serum was obtained from healthy volunteers.
Calibration Standards and Quality Control Samples
The stock solution of MTX (10,000 mM) was prepared in methanol and 0.1 N NaOH solution. Further dilution was done with methanol:water (50:50, v/v) to prepare a working solution at the desired concentration of 9.9, 19.8, 39.6, 78.12, 156.2, 312, 625, and 1,250 μM/L for calibration standard. Internal standard (IS) working solution was prepared by diluting the stock solution (10,000 μM) of aminoacetophenone (AMP) with acetonitrile–water (70:30, v/v) to a final concentration of 250 μM/L. All solutions were stored at 4°C.
Serum standard samples were prepared by mixing 10 μL of working solution into 90 μL of blank human serum to yield concentrations of 0.9, 1.98, 3.9, 7.8, 15.6, 31.2, 62.5, and 125 μM/L. Low quality control (LQC), middle quality control (MQC), and high quality control (HQC) samples were prepared from pooled serum using different stock solutions of MTX to yield a final concentration of 0.09, 0.5 (LQC) 5.0, 10 (MQC), and 12.5 μM (HQC), respectively.
Calibration standards and quality control (QC) samples were stored at −70°C until use, except that freshly prepared calibration standards were used for assessments of long-term frozen storage stability of MTX and AMP in pooled serum.
Clinical Samples
Human blood samples from ALL patients were collected in plain vacutainers (REDCap) and after coagulation, they were processed by centrifugation at ∼1,500 g (3,000 rpm) for 10 minutes at room temperature to obtain serum. The serum (500–1,000 µL) was stored in clean polypropylene tubes at 4 to 8°C till analysis.
Serum Sample Preparation for LC-MS/MS
All stored clinical study samples, calibration standards, and QC samples were thawed and then vortex mixed for ∼1 minute before pipetting. Spiked serum standard samples (100 μL) were protein precipitated using 900 μL of acetonitrile–water (70:30, v/v) containing IS (AMP, 250 μM). Blank samples were spiked with 900 μL of acetonitrile:water at 70:30, v/v). Precipitation was achieved by vortex mixing for 2 minutes. Subsequently, the mixture was centrifuged, once at 2,000 rcf for 5 minutes at 4°C. The resulting supernatant was transferred into a new 1.5 mL microcentrifuge tube without disturbing the protein precipitate. Again, it was centrifuged at 2,000 rcf for 5 minutes at 4°C to minimize any particulate matter in the solution. The supernatant aqueous solution was transferred into an autosampler vial.
Mass Spectrometry
Ionization and detection of MTX and AMP was performed on a triple quadrupole mass spectrometer (TSQ Quantis, Thermo Fisher Scientific) equipped with an electrospray ion (ESI) source in positive ion mode. MTX for peak identification and AMP (Sigma-Aldrich) was used for MS/MS optimization. Positive ions were monitored for both MTX and AMP in multiple reaction monitoring (MRM) mode. Quantification was performed using parent → product ion (m/z) to detect the transition m/z 455.1→308.3 for MTX and m/z 136.2→94.1 for IS with a dwell time of 100 milliseconds per transition (►Fig. 1). The MS conditions are given in ►Table 1. Xcalibur software version 1.4.1 was used for LC-MS/MS parameter control and data collection.
![Methotrexate and aminoacetophenone (internal standard [IS]) product ion spectrum in positive ionization mode (adapted from Wu et al, 2015[27]).](/content/164/2023/15/3/img/JLP-15-344-g001.png)
- Methotrexate and aminoacetophenone (internal standard [IS]) product ion spectrum in positive ionization mode (adapted from Wu et al, 2015[27]).
| Positive ion spray voltage | 4,000 V |
| Sheath gas (Arb) | 50 |
| Auxiliary gas (Arb) | 5 |
| Sweep gas | 1 |
| Ion transfer tube temperature | 200°C |
| Vaporizer temperature | 400°C |
| Mobile phase | A: 0.1% formic acid in water |
| B: Acetonitrile | |
| Column | C18 (3.0 μm 120Å, 2.1 × 50 mm) |
| Flow rate | 0.4 mL/min |
Ultra-High-Performance Liquid Chromatography
The LC was performed using vanquish UHPLC system (Thermo Fisher Scientific). Chromatographic separation of the compound was achieved by using the C18 column (3.0 µm 120 Å, 2.1 × 50 mm; Acculaim 120, Thermo Fisher Scientific). The mobile phase was composed of 0.1% formic acid in water (solvent A) and acetonitrile (solvent B) at a flow rate of 0.4 mL/min under room temperature. The total injection volume was 10 µL and run time for each injection was 5 minutes (►Table 2).
| Time | Flow (mL/min) | % Mobile phase A | % Mobile phase B |
|---|---|---|---|
| 0.00 | 0.400 | 40.0 | 60.0 |
| 0.50 | 0.400 | 40.0 | 60.0 |
| 3.60 | 0.400 | 80.0 | 20.0 |
| 4.10 | 0.400 | 80.0 | 20.0 |
| 4.20 | 0.400 | 40.0 | 60.0 |
| 5.00 | 0.400 | 40.0 | 60.0 |
| 5.00 | Stop run | ||
Validation of Method
The validation of the method for determination of MTX and AMP in serum was done following US-FDA Bioanalytical Method Validation Guidance for Industry, 2018.[28] The calibration, precision, accuracy, selectivity, matrix effect, injection carryover, extraction recovery, and effect of hemolysis were evaluated. Experiments were also conducted to evaluate the stability of MTX and AMP in stock solutions, samples stored at 4 to 8°C.
Calibration and Linearity
The linearity of the method was determined by analysis of standard plots associated with eight-point standard calibration curve. Eight nonzero calibration standards were analyzed in each batch of accuracy and precision. Peak area ratios of MTX:AMP obtained from MRM analysis of the chromatograms from the calibration standards and their corresponding nominal concentrations were utilized for the construction of calibration curves, using weighted (1/X2) linear least squares regression. Back calculations were made from the curve equations to determine the concentration of each analyte in each individual calibration standard sample. A correlation coefficient (r2) greater than 0.99 was required for each the calibration curve to be acceptable. The deviation of the mean back calculated concentrations of individual standards other than the lower limit of quantification (LLOQ) standard needed to be within 15.0% of the corresponding nominal concentrations for LLOQ should be within 20%.
Accuracy and Precision
Accuracy and precision of the method were evaluated by analyzing six QC sample replicates at four different nominal analyte concentrations across the standard curve range. FDA (2018) guidelines were followed for acceptance of the results of intraday and interday runs.
Dilution Integrity, Selectivity, and Matrix Effect
Dilution integrity was checked to ensure accurate measurement for samples with concentration above the upper limit of the standard curve. Serum sample was prepared at 100 µM concentration of MTX and diluted in five replicates at a dilution factor of 10 with pooled serum healthy volunteers.
The selectivity of the method was assessed by extracting and analyzing six different healthy volunteer's serum with no added MTX or AMP. Method was acceptable if the interference peak area at the retention time of the MTX was < 20.0% of the mean MTX peak area from the LLOQ and <5.0% of the mean IS peak area.
The matrix effect was determined in six different samples of different concentrations of MTX and one concentration of AMP by comparing the ratio of peak areas of solutions in the presence and absence of the matrix.
Recovery, Carryover, and Stability Assessment
The recovery of the MTX/AMP was evaluated by comparing the mean peak areas from the MTX/AMP added to and recovered from the extracted samples to the peak areas from the postextract spiked samples.
Carryover study was done by comparing the peak areas of the MTX observed in the first and second carryover blanks with the peak area observed in the lowest calibration standard.
Stability for MTX and AMP in the stock solutions that were used to prepare calibrations standards, QCs, and other validation samples was tested by comparing the response of a stock kept at −20°C to the response of a freshly prepared solution as a reference solution.
Benchtop stability of MTX in serum was evaluated in two samples by comparing the peak areas of MTX from reprocessing the sample after having left it for 8 hours in room temperature.
Comparison of LC-MS/MS and Enzyme Multiplied Immunoassay Technique-MTX Assay Methods
Paired serum samples were collected from ALL patients (n = 10) on high-dose MTX therapy in the hematology ward of our institute. One aliquot of each serum was tested using optimized LC-MS/MS method, while the other one was tested using Enzyme Multiplied Immunoassay Technique) EMIT-MTX Assay (EMIT Siemens Assay Kit, Cat# 6L119UL; Siemens Healthcare, Germany) on Siemens Viva-E analyzer according to manufacturer's instructions.
Statistical Analysis
Means, standard deviations (SDs), and values of % coefficient of variation (CV) and % relative error were calculated by standard statistical calculations using Microsoft Excel sheet. Bland and Altman plot was prepared online (https://huygens.science.uva.nl/BA-plotteR/).
Results
Method Development
Optimization of LC-MS/MS Conditions
The polar structure of MTX was ionized using ESI as the ionization source. Q1 MS full scan under positive mode produced higher sensitivity than in negative mode. Protonated molecular ions at 455.3 for MTX and 136.1 for IS were found as the predominant precursor ions in the mass spectra. We have screened for four products. Among four, 308 and 94 were found to be the most abundant and permanent product ion for MTX and IS, respectively. Thus, m/z 455.5→308.25 transition for MTX and 136.1→94.083 transition for IS were used for further quantitative detection (►Fig. 2A, B).
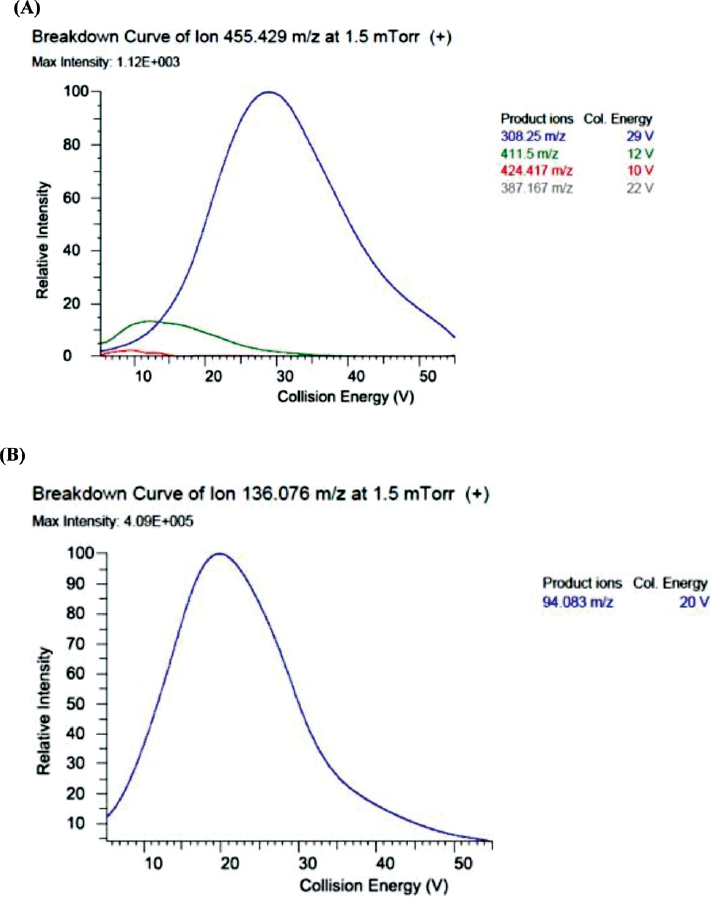
- Optimized product ion spectrum and their intensity of internal standard and methotrexate.
Chromatographic separation of MTX using the C18 column showed a retention time of 0.33 for MTX and 0.48 for IS with a sharp and symmetrical peak. Acetonitrile was selected as an organic mobile phase because it provided a better chromatographic shape than methanol. The intensity of MTX obtained by adding 0.1% formic acid in the mobile phase was higher when compared with 0.03% acetic acid (►Fig. 3A, B). An injection volume of 10 μL was adequate to acquire good sensitivity and reproducibility.
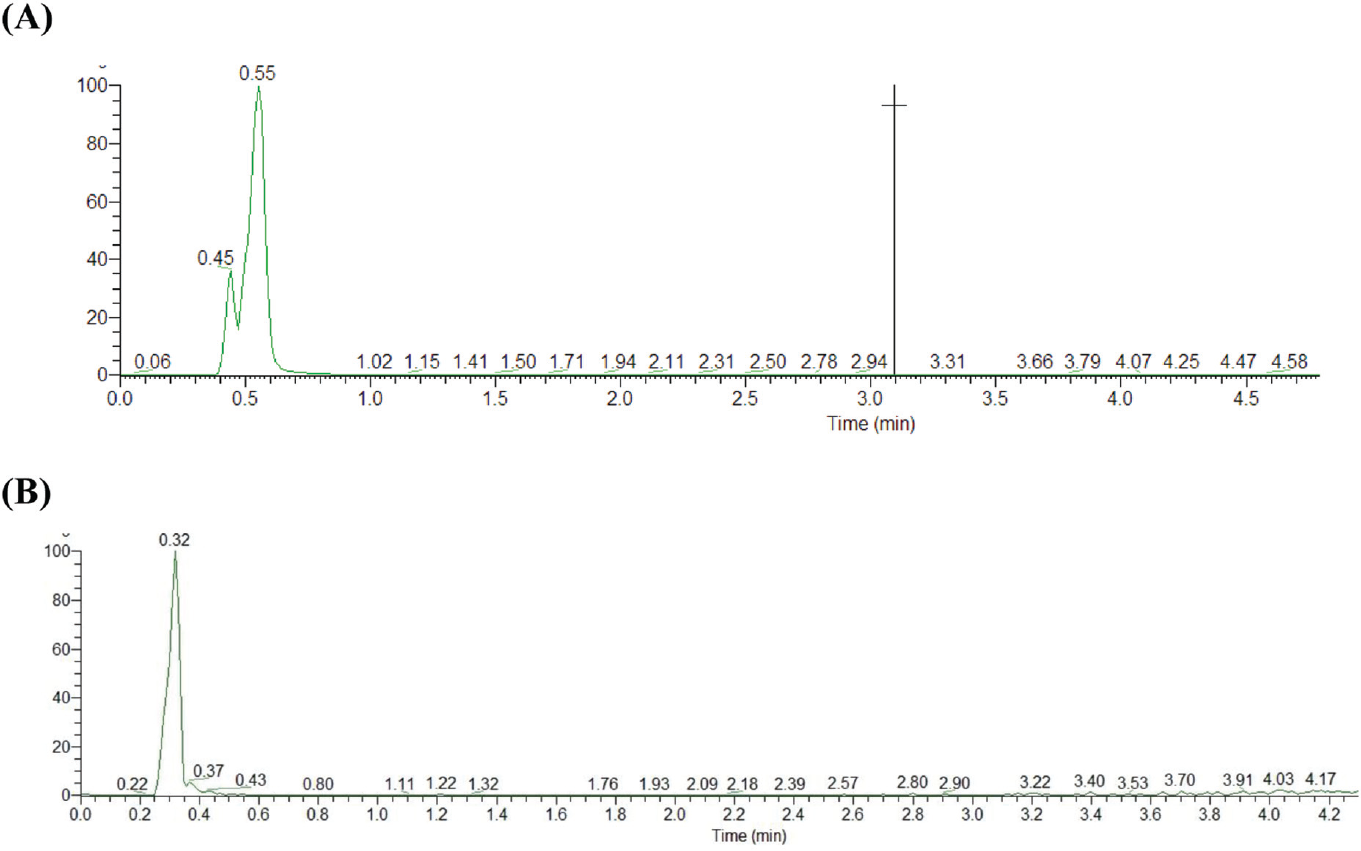
- (A) 0.03% acetic acid as mobile phase A and (B) 0.01% formic acid as mobile phase A.
Optimization of Sample Preparation
Various studies have reported different methods for MTX extraction including solid-phase extraction, liquid–liquid extraction, and protein precipitation. The protein precipitation method is quick and was found to be efficient for therapeutic drug monitoring. Organic solvents such as methanol and acetonitrile were most commonly used as protein precipitants for LC-MS/MS. The best signal and intensity of MTX were obtained by precipitation with acetonitrile–water in a ratio of 70:30 followed by two times centrifugation at 2,100 rcf for 5 minutes at 4°C. The precipitating solution was spiked with IS (AMP).
Method Validation
The quantitation range for MTX was validated from 0.09 to 25 µM. The precision and accuracy of the method for determination of MTX in serum were evaluated by analyzing QC samples at four levels (LLOQ: 0.09 µM, low LQC: 0.5 µM, MQC: 5.0 and 10.0 µM, and HQC: 12.5 µM) within the range of calibration standard. The results of precision and accuracy observed in six sample runs were within the acceptance criteria. All the validation assessments such as calibration and linearity, specificity, carryover, matrix effect, and extraction recovery were also passed the acceptance criteria, and the results are shown in ►Table 3.
| 1 | Analyte name | Methotrexate |
| 2 | Internal standard | Aminoacetophenone |
| 3 | Analytical method type | LC-MS/MS |
| 4 | Extraction method | Protein precipitation |
| 5 | Sample volume | 10 μL |
| 6 | QC concentrations | 0.09, 0.5, 5, and 10 μM |
| 7 | Standard curve concentrations | 0.09, 0.198, 0.39, 0.78, 1.56, 3.12, 6.25, and 12.5 μM |
| 8 | Lower limit of quantitation | 0.09 |
| 9 | Upper limit of quantitation | 12.5 |
| 10 | Mean recovery of analyte (%) | 40 |
| 11 | Mean recovery of IS (%) | 40 |
| 12 | LLOQ QC intraday precision range (% CV) | 10.25–12.5 |
| 13 | LLOQ QC intraday accuracy range (% RE) | 11.1–18.9 |
| 14 | Analytical QC intraday precision range (% CV) | 1.0–2.18 |
| 15 | Analytical QC intraday accuracy range (% RE) | 0.01–15.2 |
| 16 | LLOQ QC interday precision range (% CV) | 10.25–19.4 |
| 17 | LLOQ QC interday accuracy range (% RE) | 11.1–23.3 |
| 18 | Analytical QC interday precision range (% CV) | 1.6–20 |
| 19 | Analytical QC interday accuracy range (% RE) | 79–103 |
| 20 | Dilution integrity | 1:5 in water |
| 21 | Selectivity at LLOQ | 86% for MTX and 99.7% for IS |
| 22 | Matrix effect | 4.56% for low QC and 2.1% for high QC |
Abbreviations: CV, coefficient of variation; IS, internal standard; LC-MS/MS, liquid chromatography–tandem mass spectrometry; LLOQ, lower limit of quantification; QC, quality control; RE, relative error.
Linearity and Lower Limit of Quantification
Eight calibration standards were validated over the concentration range of 0.09 to 12.5 μM. The correlation coefficient of all the calibration standards was >0.99, which showed good linearity. The mean linear regression equation of calibration standards during the validation was: Y = 0.000689623 + 0.0253818 × X, r = 0.9928, where Y represents the peak area ratio of MTX to IS and X represents the nominal concentration of MTX. The LLOQ for MTX was determined to be 0.09 μM/L according to the clinical requirement with accuracy and precision (►Fig. 4 and ►Table 4).

- (A) The correlation coefficient of 10 calibration standards was >0.99, which showed good linearity and (B) showed the peak of the lower limit of quantification (0.09 µM/L).
| Parameters | Lower limit of Quantification | Low quality control | Medium quality control | High quality control | |
|---|---|---|---|---|---|
| Concentration (µM/mL) | 0.09 | 0.426 | 5.218 | 10.031 | 12.455 |
| 0.08 | 0.435 | 5.151 | 10.119 | 12.556 | |
| 0.077 | 0.424 | 5.123 | 10.107 | 12.499 | |
| 0.073 | 0.429 | 5.22 | 9.999 | 12.696 | |
| 0.069 | 0.426 | 5.408 | 9.727 | 11.978 | |
| Mean | 0.0778 | 0.428 | 5.224 | 9.996 | 12.436 |
| Standard deviation | 0.007981 | 0.004301 | 0.1111 | 0.1589 | 0.2720 |
| Precision (% CV) | 10.258 | 1.004 | 2.128 | 1.589 | 2.18 |
| Nominal value (µM/mL) | 0.09 | 0.50 | 5.0 | 10.0 | 12.50 |
| Accuracy (%) | 113.5 | 114.4 | 95.5 | 100 | 100.5 |
| Number of samples (n) | 5 | 5 | 5 | 5 | 5 |
Abbreviations: CV, coefficient of variation; LC-MS/MS, liquid chromatography–tandem mass spectrometry.
Selectivity
Six blank serums from different individuals were run to evaluate to select the peak of analytes and specificity in retention time. ►Fig. 5A shows chromatograms of blank serum, ►Fig. 5B shows blank serum spiked with MTX at a (LLOQ: 0.09 µM), and ►Fig. 5C shows serum from a patient 24 hours after administration of MTX, serum spiked with IS. The retention time for MTX and IS were 0.34 and 0.46, respectively (►Fig. 5).
![Chromatograms of methotrexate (MTX) and p-aminoacetophenone (internal standard [IS]) in human serum: (A) blank serum; (B) blank serum spiked with MTX at 0.09 μmol/L and IS at 250 μmol/L; and (C) chromatograms of MTX and IS in a serum sample from a patient 24 hours after administration of MTX.](/content/164/2023/15/3/img/JLP-15-344-g005.png)
- Chromatograms of methotrexate (MTX) and p-aminoacetophenone (internal standard [IS]) in human serum: (A) blank serum; (B) blank serum spiked with MTX at 0.09 μmol/L and IS at 250 μmol/L; and (C) chromatograms of MTX and IS in a serum sample from a patient 24 hours after administration of MTX.
Carryover
LC autosampler injection carryover was evaluated by injection of a blank serum sample immediately after the injection of the highest calibration standards. The carryover was measured as the peak area observed in the carryover blank expressed as a percentage of the mean peak area of the lowest calibration standard determined in the same run. The carryover value observed was 10% of the mean LLOQ analyte peak area which was within the acceptance criteria <20%.
Matrix Effect
The matrix effect was determined in six different individual lots of serum at low and high (0.09, 12.5 μM/L) QCs. Six aliquots of each low and high QCs of each matrix were analyzed using freshly processed calibration curve samples. The % CV of the results for the mean IS-normalized matrix factor were 4.56 and 2.1%, respectively, which is within the acceptance range <15%.
Extraction Recovery
The extraction recovery was evaluated by comparing the mean area of six replicates at low (0.09 µM), medium (0.78 µM), and high concentration (12.5 µM) of MTX and one concentration of ISD (250 µM) in serum spiked and water spiked. The results show 40% recovery, which is consistent and proportionate to the recovery of the ISD.
Stability of Different Concentrations of MTX Calibrators
The stability of MTX calibrators (0.09–12.5 μM/L) and IS (250 μM/L) spiked in serum was evaluated by comparing the peak area ratio of serum unspiked MTX calibrators after storage at room temperature for 8 hours and at 4°C for 24 hours; >90% stability was obtained at both the temperature and time.
Comparison of Methods
The results of LC-MS/MS method and those of commercial immunoassay (EMIT-MTX Assay) in sera of ALL patients were compared using Bland–Altman analysis. The X-axis is the mean of measurement of these methods, and Y-axis is the difference between the two measurements. The results from both methods showed <1.4 SD (►Fig. 6).
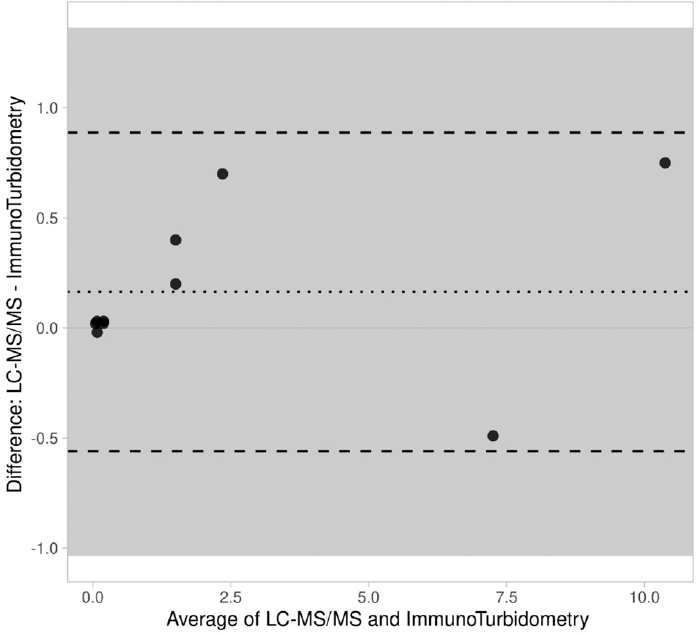
- Bland–Altman plot for methotrexate measured with LC-MS/MS and immunoturbidity. The difference between the two methods is plotted against the average of the two methods. LC-MS/MS, liquid chromatography–tandem mass spectrometry.
Discussion
Cancer being a leading cause of death has a major impact on public health worldwide. MTX, a structural analog of folate, is the most effective drug extensively used in clinical practice for treating various types of cancers including ALL, osteosarcoma, and lymphomas. A high dose (>1 g/m2) of MTX in multiple doses and schedules is particularly used for the treatment of hematologic as well as solid cancers.[3,4,5] However, MTX is poorly metabolized and most of the part is present in its native form which can induce life-threatening adverse effects. Thus, after its high-dose administration, serum levels of MTX in cancer patients must be precisely monitored at the fixed time points to improve treatment outcome and avoid severe side effects in clinical settings.
In this study, we developed a LC-MS/MS based method for the quantification of MTX levels in human serum as it is a simple, rapid, and highly sensitive platform for therapeutic drug monitoring without using any chromophore or fluorophore. Our sample preparation was based on protein precipitation, which is the simplest sample preparation approach involving addition of a precipitating solvent (acetonitrile), vortexing, and centrifugation steps only. We achieved a rapid chromatographic separation using C18 UHPLC column and gradient elution with mobile phase of 0.1% formic acid aqueous solution and Acetonitrile at a flow rate of 0.4 mL/min for 5 minutes at room temperature. MS detection was performed in a triple-quadrupole tandem mass spectrometer under positive ion mode. The assay run time of our method was 5 minutes. We prepared 10 calibration standards and spiked into human serum with a final concentration range of 0.09 to 50 µM, the correlation coefficient of all the 10 calibration standards was >0.99, which showed good linearity similar to method of Wu et al (2015).[27] In our method, the mass transitions for MTX and IS were m/z 455.5→308.25 and 136.1→94.083, respectively, as the most abundant and permanent product ion similar to that of Wu et al's method.[27]►Fig. 4(A to C) shows the specificity of retention time of MTX and IS. The blank human serum did not show a peak of MTX and IS, while human serum spiked with the lowest concentration of MTX (0.09 µM) and IS (9 µM), and the patient serum after 24-hour infusion of MTX showed consistent retention time 0.33 minutes for MTX and 0.48 minutes for IS. The precision and accuracy were determined by injecting one standard (25 µM) five times, the accuracy and precision was 23.40 ± 1.2% and 95%, respectively. The 0.09 µM LLOQ of MTX observed in our method was greater than that of Wu et al's method (0.05 µM) and recently developed LC-MS/MS assay for serum MTX by McTaggart and Keevil (0.03 µM).[27,29] However, this will not affect the clinical decision in monitoring of MTX drug concentration in serum. Our method is a rapid one with less total run time from injection to injection as compared with method of Wu et al.[27] In addition, these assays have used HPLC system, while in our assay, we have used UHPLC system, which is the latest system available to date.
Commercially available EMIT-MTX assay is a homogeneous enzyme immunoassay commonly used in clinical laboratories in India for quantitative analysis of MTX in human serum or plasma.[20] We used our LC-MS/MS method and this commercial immunoassay to determine the MTX levels in paired sera of ALL patients receiving high dose of the drug and compared the results of both the methods. The Bland–Altman plot analysis showed no significant deviation between the results of the two methods. Moreover, the serum MTX levels obtained from our LC-MS/MS method also had a good correlation with the clinical status of the ALL patients. All these facts collectively show that LC-MS/MS method developed by us would be useful for optimizing/individualizing MTX dosing for therapy, reducing drug toxicity, and improving the treatment outcome in clinical practice.
Conclusion
We were able to develop and validate not only a rapid and sensitive LC-MS/MS method offering unequivocal quantification of MTX in human serum without any analytical interference from the drug metabolites but also a cost effective as we have utilized AMP as an IS rather than costlier heavy isotope of MTX. The application of this method on sera from ALL patients on high MTX therapy shows a good clinical correlation. This method could successfully be applied as routine patient care test in the tandem LC-MS equipped clinical laboratories for therapeutic drug monitoring of MTX in ALL and other cancer patients.
Conflict of Interest
None declared.
References
- Folate metabolism: a re-emerging therapeutic target in haematological cancers. Leukemia. 2021;35(06):1539-1551.
- [CrossRef] [PubMed] [Google Scholar]
- The methotrexate story: a paradigm for development of cancer chemotherapeutic agents. Adv Enzyme Regul. 1994;34:397-419.
- [CrossRef] [PubMed] [Google Scholar]
- High-dose methotrexate-based regimens and post-remission consolidation for treatment of newly diagnosed primary CNS lymphoma: meta-analysis of clinical trials. Sci Rep. 2021;11(01):2125.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and association analysis of high-dose methotrexate in the treatment of children with acute lymphoblastic leukemia. Oncol Lett. 2019;17(05):4423-4428.
- [CrossRef] [Google Scholar]
- High-dose methotrexate therapy significantly improved survival of adult acute lymphoblastic leukemia: a phase III study by JALSG. Leukemia. 2018;32(03):626-632.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and management of patients with methotrexate delayed elimination in the clinical practice: a Delphi study. J Oncol Pharm Pract. 2022;10 781552221079568
- [Google Scholar]
- Renal function and plasma methotrexate concentrations predict toxicities in adults receiving high-dose methotrexate. Med Sci Monit. 2018;24:7719-7726.
- [CrossRef] [PubMed] [Google Scholar]
- Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21(12):1471-1482.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for hepatic toxicity of high-dose methotrexate in patients with osteosarcoma. Anticancer Res. 2022;42(02):1043-1050.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma homocysteine, methionine and S-adenosylhomocysteine levels following high-dose methotrexate treatment in pediatric patients with acute lymphoblastic leukemia or Burkitt lymphoma: association with hepatotoxicity. Leuk Lymphoma. 2014;55(07):1591-1595.
- [CrossRef] [PubMed] [Google Scholar]
- A review of methotrexate-associated hepatotoxicity. J Dig Dis. 2014;15(10):517-524.
- [CrossRef] [PubMed] [Google Scholar]
- Late effects of high-dose methotrexate treatment in childhood cancer survivors-a systematic review. BMC Cancer. 2022;22(01):267.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate neurotoxicity is related to epigenetic modification of the myelination process. Int J Mol Sci. 2021;22(13):6718.
- [CrossRef] [PubMed] [Google Scholar]
- Neurotoxicity after high-dose methotrexate (MTX) is adequately explained by insufficient folinic acid rescue. Cancer Chemother Pharmacol. 2017;79(06):1057-1065.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate in sarcoidosis: hematologic and hepatic toxicity encountered in a large cohort over a six year period. Sarcoidosis Vasc Diffuse Lung Dis. 2020;37(03):e2020001.
- [Google Scholar]
- Adverse effects with intravenous methotrexate in children with acute lymphoblastic leukemia/lymphoma: a retrospective study. Indian J Hematol Blood Transfus. 2020;36(03):498-504.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate pulmonary toxicity: deep inspiration. Arthritis Rheumatol. 2020;72(12):1959-1962.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate-induced pneumonitis: heterogeneity of bronchoalveolar lavage and differences between cancer and rheumatoid arthritis. Inflamm Allergy Drug Targets. 2014;13(01):25-33.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate pulmonary toxicity. Expert Opin Drug Saf. 2005;4(04):723-730.
- [CrossRef] [PubMed] [Google Scholar]
- Modified enzyme multiplied immunoassay technique of methotrexate assay to improve sensitivity and reduce cost. BMC Pharmacol Toxicol. 2019;20(01):3.
- [CrossRef] [PubMed] [Google Scholar]
- Analytical methodologies for determination of methotrexate and its metabolites in pharmaceutical, biological and environmental samples. J Pharm Anal. 2019;9(06):373-391.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of clinical assays for measuring high-dose methotrexate in plasma. Clin Chem. 1996;42(01):39-44.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic drug monitoring of methotrexate in plasma using ultra high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry: necessary after administration of glucarpidase in methotrexate intoxications. Ther Drug Monit. 2018;40(04):383-385.
- [CrossRef] [PubMed] [Google Scholar]
- Analytical interference in the therapeutic drug monitoring of methotrexate. Ann Biol Clin (Paris). 2016;74(03):333-337.
- [CrossRef] [PubMed] [Google Scholar]
- DAMPAned methotrexate: a case report and review of the management of acute methotrexate toxicity. Can J Kidney Health Dis. 2019;6 20 54358119895078
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate polyglutamates analysis by chromatography methods in biological matrices: a review. Anal Sci. 2021;37(12):1655-1664.
- [CrossRef] [PubMed] [Google Scholar]
- A simple, rapid and reliable liquid chromatography-mass spectrometry method for determination of methotrexate in human plasma and its application to therapeutic drug monitoring. Biomed Chromatogr. 2015;29(08):1197-1202.
- [CrossRef] [PubMed] [Google Scholar]
- Bioanalytical Method Validation Guidance for Industry. 2018 Accessed at: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf
- [Google Scholar]
- A rapid LC-MS/MS assay for the measurement of serum methotrexate in patients who have received high doses for chemotherapy. Ann Clin Biochem. 2021;58(06):599-604.
- [CrossRef] [PubMed] [Google Scholar]






