Translate this page into:
Validated ultraviolet high-performance liquid chromatography method for post-mortem 5-hydroxy-indoleacetic acid measurement in human cerebrospinal fluid
The study was approved by Institutional human ethics committee of AIIMS Bhopal vide LOP letter no 2020/PG/Jan/09 dated Nov 21, 2020.
-
Received: ,
Accepted: ,
How to cite this article: Bhushan D, Yadav J, Arora A, Agrawal D, Apte A. Validated ultraviolet high-performance liquid chromatography method for post-mortem 5-hydroxy-indoleacetic acid measurement in human cerebrospinal fluid. J Lab Physicians, doi: 10.1055/s-0043-1774378
Abstract
Objective:
The objective of this study was to develop an ultraviolet high-performance liquid chromatography (UV-HPLC) method for the measurement of 5-hydroxyindoleacetic acid (5-HIAA) in human cerebrospinal fluid (CSF) as a potential biomarker for neurological and psychiatric illnesses, including depressive disorders with suicidal behavior.
Methods:
The study utilized CSF samples from individuals brought for medicolegal autopsy. The 5-HIAA concentration was measured using a UV-HPLC method with three mobile-phase solvents. The most effective mobile phase solvent was then used to measure 5-HIAA in the CSF samples.
Materials:
The materials used in the study included CSF samples obtained from individuals brought for medico-legal autopsy, UV-HPLC equipment, and mobile phase solvents, including 5-hydroxyindole-3-acetic acid (Merck/sigma), acetonitrile, concentrated formic acid, concentrated acetic acid, methanol, and phosphoric acid.
Statistical Analysis:
This was done using R Studio (version 4.2.0).
Results:
The study found that the UV-HPLC method utilizing formic acid (0.05–0.1%): acetonitrile in a 1:9 as mobile phase was the most effective for measuring 5-HIAA in human CSF. The method exhibited excellent linearity, accuracy, and precision.
Conclusion:
The study concludes that the developed UV-HPLC method is reliable and effective for measuring 5-HIAA in human CSF. Measuring 5-HIAA levels in CSF can serve as a potential biomarker for neurological and psychiatric illnesses, including depressive disorders with suicidal behavior. This method is promising for clinical and forensic practice to diagnose suicidal cases. Further research is needed to determine the clinical significance of these findings and the potential for broader application in psychiatry. This article helps to give a practical, cost-effective methodology to detect CSF 5-HIAA.
Keywords
5-hydroxyindoleacetic acid
cerebrospinal fluid
high-performance liquid chromatography (HPLC)
suicidal
INTRODUCTION
The cerebrospinal fluid (CSF) is a natural route by which many transmitter metabolites leave the brain.[1] CSF is a clear fluid formed as an ultrafiltrate of plasma. The spinal and intracranial compartments bothcontain CSF. It circulates along CSF channels in the subarachnoid space of the brain and spinal cord. The choroid plexus continually secretes it consistently inside the brain’s ventricles. The average adult’s CSF contains approximately 140 mL and 500 to 700 mL of CSF is secreted daily, or 0.2 to 0.7 mL per minute. The primary function of the CSF is to reduce the buoyancy of the brain. Additionally, it provides nutrients and helps eliminate several substances, including cells, neurotransmitters, amino acids, and metabolic waste materials. Several studies have suggested that CSF metabolites, in particular homovanillic acid and 5-hydroxyindoleacetic acid (5-HIAA), reflect the turnover of their parent amines in the brain.[2,3] 5-HIAA is the final metabolite of serotonin.[4] The CSF compartment, which is anatomically connected to the brain, is a valuable source for tracking the number of vital biomarkers that are known to have a role in the pathophysiology of central nervous system illnesses[5] and suicidal cases.[6] Reversed-phase HPLC, microdialysis, positron emission tomography tracers, etc., are widely used methods for analyzing the metabolites in CSF.[7] Measurement of serotonin metabolite in CSF is used for the diagnosis of various neurological and psychiatric illnesses.[8] Analysis for CSF from autopsy samples requires unique methods for collection and analysis. This study involves the development of the ultraviolet highperformance liquid chromatography (UV-HPLC) method for the estimation of CSF 5-HIAA, along with the collection and optimization of the sample preparation technique. For the measurement of ventricular 5-HIAA, we have used HPLC with UV detection using Waters 2489 UV/Visible detector.
MATERIALS
Autopsy instruments, 10 mL syringe with needle, 5 mL Eppendorf tubes, centrifugation machine, Acrodisc sterile syringe filter having a pore size of 0.22 µm and 25 mm diameter (Pall Corporation, Pall Membrane Technology Center. 770 Pennsylvania Drive, Suite 100. Exton, Pennsylvania, United States), compatible freezer (-80oC), 5-hydroxyindole-3-acetic acid (Merck/sigma), acetonitrile(Sigma-Aldrich, United States), concentrated formic acid, concentrated acetic acid, methanol, phosphoric acid, ultrafilter (Ultra 370 series RIONS India), electronic balance machine, 1,000 mL measuring cylinder, micropipettes, HPLC (Waters 2489 UV/Visible detector).
Collection of CSF
CSF was collected by direct visualization method during autopsy. After opening the skull and removing the dura, under direct vision, the two cerebral hemispheres are slightly parted using the index and middle fingers. Then a sterile needle with 10 mL syringe attached to it was inserted into the dependent and posterior part of the brain’s lateral ventricle at around 1.5 cm depth. The needle was directed posteriorly and downward in each ventricle, and CSF was aspirated (Figure 1). Around 4 to 5 mL of CSF was collected from the brain’s lateral ventricles and transferred to the 5 mL Eppendorf tubes. Then the CSF was centrifuged upon collection at 15,000 rpm for 15 minutes at 4 °C and filtered with a sterile nylon syringe filter with a pore size 0.22µm. Then CSF sample was stored at —80°C freezer until analysis.
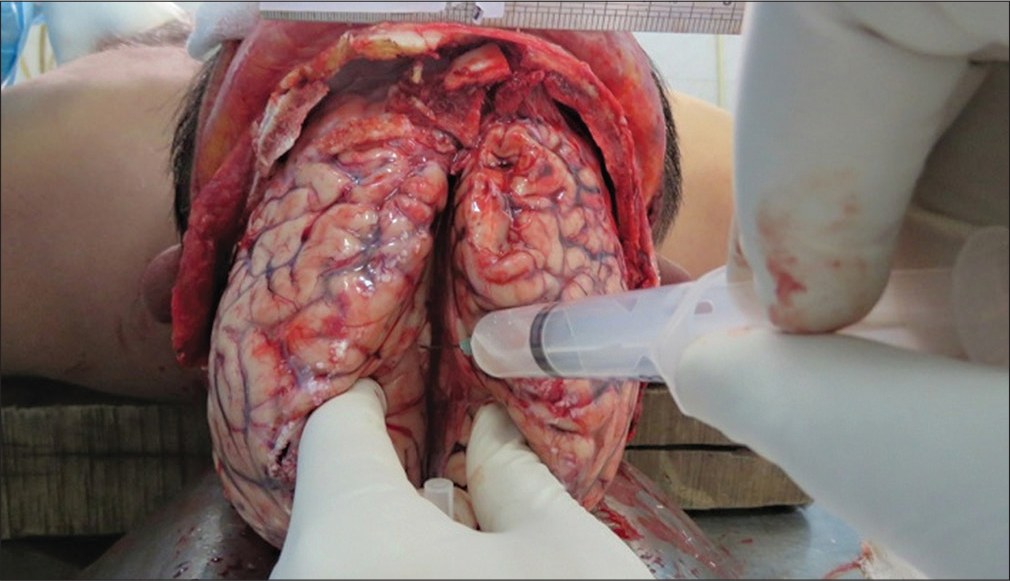
- Collection of cerebrospinal fluid during autopsy after opening the skull.
UV-HPLC Method Development for 5-HIAA
HPLC analysis to detect the 5-HIAA in CSF samples was performed using an HLPC pump system (Waters 515) fitted with Waters 2489 UV/visible detector and reversed-phase SunFireTM C-18 column, 5 μm, 4.6 × 25 mm was used. The sample auto-injector used was fitted with an in-line degasser. The screening of different mobile phases, including (acetic acid [0.01–0.1%]: methanol in 1:9 ratio), (phosphoric acid [0.01–0.1%]: methanol in 1:9 ratio) and (formic acid [0.05–0.1%]: acetonitrile in 1:9 ratio), was performed at flow rate ranging from 0.5 mL/min to 1.5 mL/min. The resolution of 5-HIAA was performed at three different wavelengths, including 220, 280, and 320 nm. The mobile phase containing formic acid (0.05%) and acetonitrile in 1:9 at flow rate of 1.0 mL/min determined at 280 nm was found to be optimum to resolve the peak of 5-HIAA with a running time of 20 minutes at various dilution of 50, 100, 150, and 200 nm (Figure 2); these parameters obtained were further used to analyze all the clinical samples. The peak from other mobile phases (acetic acid [0.01–0.1%]: methanol in 1:9 ratio), (phosphoric acid [0.01–0.1%]: methanol in 1:9 ratio) did not yield satisfactory results.
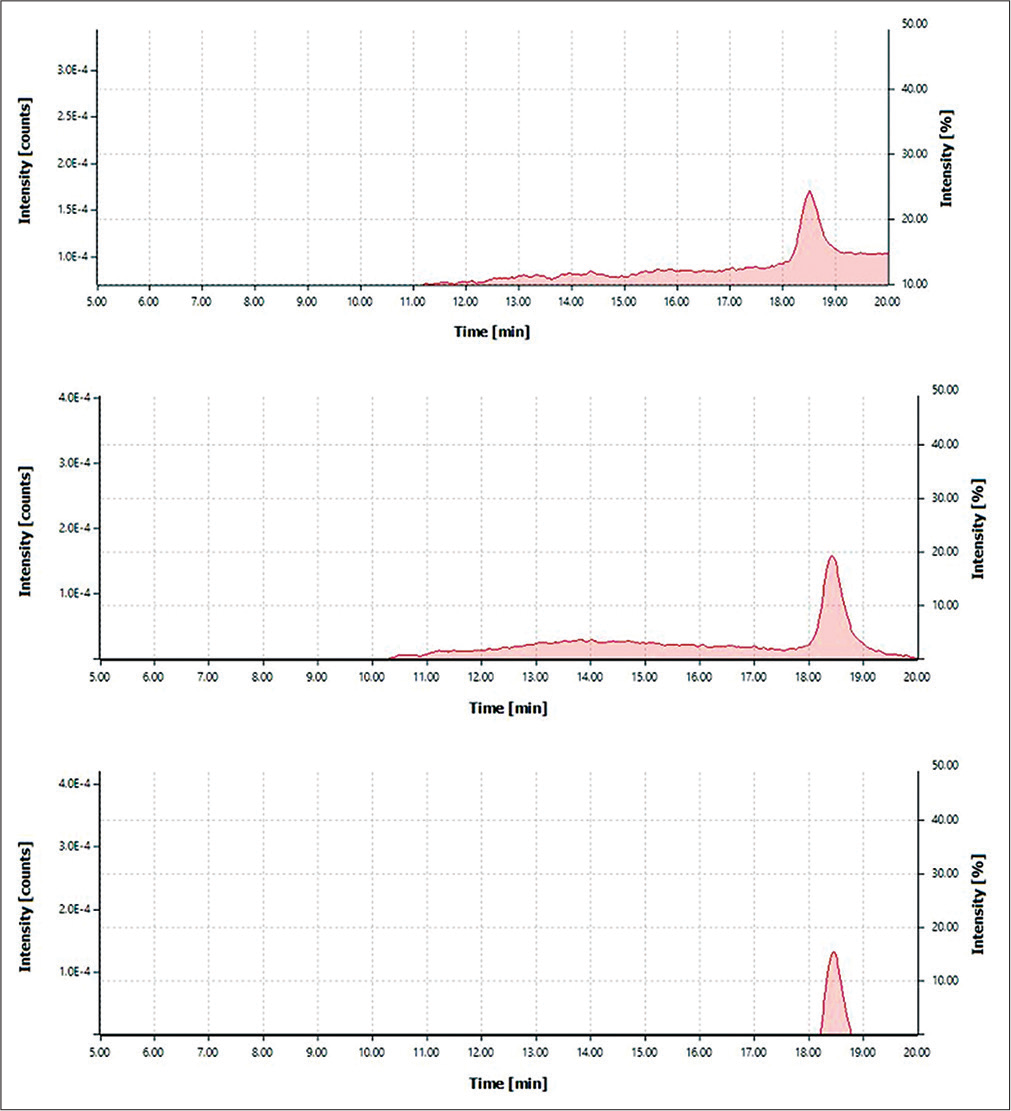
- 5-hydroxyindoleacetic acid concentration graph at 100, 150, and 200 nm.
Analysis of CSF 5-Hydroxyindoleacetic Acid by HPLC
CSF samples were removed from the deep freezer (—80°C) and subjected to thawing on ice (4°C). After thawing, the CSF samples were centrifuged again at 15,000 rpm at 4°C for 15 minutes and filtered using a syringe filter (Pall Corporation, United States); 90 µL of this filtered CSF sample was directly injected into a reversed-phase HPLC column fitted with UV detector, using an auto-injector.
The standard solution of 100 nm 5-HIAA (Merck, India) was used to calculate the concentration of 5-HIAA in all the clinical samples. All the runs were performed in triplicates to check the repeatability of the results. We calculated the CSF 5-HIAA concentration from HPLC analysis quantitatively by comparing it with the concentration of the standard sample. A comparison of the area under the curve for the unknown sample (cases) and that of the known sample was used to determine the amount of CSF 5-HIAA in samples.
Statistical Analysis
All the statistical analysis was performed using R Studio (version 4.2.0). Continuous data were expressed as mean, standard deviation, or median (interquartile range) depending on the distribution.
RESULTS
The study was approved by our institute’s Institutional Human Ethics Committee vide LOP letter no 2020/PG/ Jan/09. A total of 71 CSF samples were collected for 5-HIAA assessment from 71 dead bodies after obtaining due consent from the next of kin. Out of the three mobile phases used for UV-HPLC, the one containing formic acid (0.05%) and acetonitrile in 1:9 at a flow rate of 1.0 mL/min determined at 280 nm was found to be optimum to resolve the peak of 5-HIAA with a running time of 20 minutes at various dilution of 50, 100, 150, and 200 nm. The peak with the standard solution was obtained at 18.4 minutes at different dilutions mentioned. This mobile phase was then used for the quantification of the 5-HIAA level from the CSF in all the samples.
Out of the 71 cases studied, the median of 5-HIAA level in CSF was found to be 97.46 nMol/L with an interquartile range of 80.86 to 114.97 nMol/L. The minimum level of 5-HIAA in CSF was 12.85 nMol/L, and the maximum level of 5-HIAA in CSF was 527.51 nMol/L.
DISCUSSION
The present work contributes a new and straightforward method for estimating a serotonin metabolite, that is, 5HIAA, in CSF samples using UV-HPLC. The normal psychotic function is disturbed by the fluctuation of neurotransmitters since serotonin plays a significant part in sustaining ordinary psychosis.[7,8] A change in the concentrations of brain serotonin in disorders like depression, schizophrenia, panic disorder, and Parkinson’s disease is already established,[8] indicating the need for developing simple, sensitive, and reliable methods for estimating serotonin metabolite in CSF samples. We used a sample of CSF to determine the amount of serotonin since the level of serotonin in blood differs from that in the brain.[9] CSF, being in close proximity to the brain and a clear fluid with less protein content,[10] is the best-suited biological fluid for the estimation of 5-HIAA by HPLC. However, the relevant literature on estimating 5-HIAA in biological samples using UV-HPLC is limited. The CSF neurotransmitter composition is consistent under normal circumstances. However, the composition, quantity, and pressure can change in a variety of neurological diseases. Since serotonin degrades quickly to 5-HIAA, this parameter is used to measure the brain’s serotonin levels.[11] There are advanced methods like liquid chromatography-mass spectrometry, HPLC with fluorescence detection,[12] HPLC with electrochemical detection, gas chromatography-mass spectrometry, enzyme-linked immunosorbent assay, and capillary electrophoresis, which can detect these compounds at lower levels.[13] However, it requires expensive equipment, solvents, and extensive sample preparation. Although these methods are faster and more sensitive than UV-HPLC,[14,15] they require more expensive instruments, preparation, and skill and are only feasible in high-end laboratories. Although this study did not focus on assessing the sensitivity of this method, there are studies in the literature with a detection limit of 0.2 mg/L in 5-HIAA by HPLC in urine.[16] In our study, the minimum value of 12.85 nMol/L was detected in one of the samples with a history of depression, which is comparable.
UV-HPLC is a sensitive method that detects CSF 5-HIAA levels quickly with precision and accuracy, using inexpensive chemicals and requires significantly less sample preparation. We used three different sample preparation methods, and the best one was ascertained based on the accuracy of the result using a standard solution of 5-hydroxyindole-3-acetic acid (Merck/sigma).
During the analysis of 5-HIAA with HPLC, many problems like baseline drift, poor peak resolution, ghost peak, and system leak were faced. Contamination in the sample is also a big problem, which can interfere with the accuracy and specificity of the measurements. The possible sources of contamination include impurities in the solvents or reagents, poor cleaning or sterilization of the equipment or containers, cross-contamination from other samples or sources, and poor sample handling or storage. To avoid contaminationrelated issues, high-quality solvents and reagents were used, and the equipment was cleaned and sterilized by flushing the equipment and containers thoroughly and adequately and handling the samples carefully. The CSF samples were centrifuged just after collection and once more before analysis. Centrifugation removes particulate matter such as cells, debris, and other insoluble material from the sample, which can clog the HPLC column and affect the accuracy of the results. It also removes large molecules, such as proteins or lipids, that may interfere with separating the analytes of interest in the HPLC column.[17] This improved the sensitivity and reproducibility of the analysis. To avoid or resolve problems in UV-HPLC, it is crucial to maintain the system and components, follow proper operating procedures, and optimize the experimental conditions.
Numerous illnesses exhibit a pronounced decrease in 5HIAA levels, according to research that has been undertaken. Decreased CSF serotonin levels have also been associated with suicidal deaths[6] and can be further researched to corroborate suicidal deaths. Serotonin is thus a possible biomarker for many neurological and psychiatric illnesses, opening more possibilities for future research into the creation of alternative biomarkers in forensics and medicine.
CONCLUSION
A novel, sensitive, precise, and reliable UV-HPLC method for separating and quantifying 5-HIAA in human CSF samples has been developed. CSF is a suitable sample type for the 5-HIAA measurement by UV-HPLC method and can be used in diagnosing and monitoring patients with neuroendocrine tumors and various psychiatric diseases. Their use in this context should be adopted routinely as an alternative to other methods. Commercial kits and methods other than UV-HPLC for analyzing 5-HIAA require extensive sample preparation. In contrast, using UV-HPLC, the serotonin metabolites, that is, 5-HIAA, can be measured in a quick analysis. This method applies to clinical 5-HIAA UV-HPLC analysis in CSF and provides the benefits of both fast analysis times and low-cost sample preparation procedures.
Acknowledgments
The authors would like to thank all the department staff & technicians of the department for helping with the sample collection. Our heartfelt tribute to all the departed souls who got Nirvana from their lives.
Authors ’ Contributions
D.B. helped in inception of idea and carrying out the work and manuscript preparation. J.Y. was responsible for inception of idea, guidance, and supervision and writing of the manuscript. A.A. helped in supervision and facilitation for work and editing of the manuscript and carrying out the laboratory work and analyzing data. D.A. was involved in guidance and supervision in analyzing the data.
Conflict of Interests
The author(s) declared no potential conflicts of interest concerning the research, authorship, and publication of this article.
Funding
The author(s) disclose receipt of the following financial support for this article’s research, authorship, and publication: ICMR MD/MS financial thesis support.
References
- Cerebrospinal Fluid. Clinical Biochemistry of Domestic Animals. Elsevier Publisher; 2008. p. :769-819.
- [CrossRef] [Google Scholar]
- Reduced cerebrospinal fluid 5-hydroxy indole acetic acid and homovanillic acid in children with epilepsy. Neurology. 1975;25:72. Doi: 10.1212/WNL.25.1.72
- [CrossRef] [PubMed] [Google Scholar]
- Cerebrospinal fluid concentrations of biogenic amines and corticotropin-releasing factor in adolescent non-human primates as a function of the timing of adverse early rearing. Stress. 2002;5:185-193.
- [CrossRef] [PubMed] [Google Scholar]
- Biochemistry, 5 Hydroxyindoleacetic Acid In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. 2022 Jan-
- [PubMed] [Google Scholar]
- A novel unbiased proteomic approach to detect the reactivity of cerebrospinal fluid in neurological diseases. Mol Cell Proteomics. 2011;10:0042.
- [CrossRef] [PubMed] [Google Scholar]
- CSF 5-HIAA, attempted suicide and suicide risk in schizophrenia spectrum psychosis. Schizophr Res. 2009;112(1-3):80-85.
- [CrossRef] [PubMed] [Google Scholar]
- 5-HIAA as a potential biological marker for neurological and psychiatric disorders. Adv Pharm Bull. 2019;9:374-381.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation Between Salivary, Platelet and Central Serotonin Levels in Children | Canadian Journal of Neurological Sciences | Cambridge Core [Internet] [cited 2022 Nov 3]. Accessed August 18, 2023 at: https://www.cambridge.org/core/journals/canadian-journal-of-neurologicalsciences/article/correlation-between-salivary-platelet-and-central-serotonin-levels-inchildren/98A7C394671DBF747CF97E88B944C6D2
- [Google Scholar]
- Parallel changes in serotonin levels in brain and blood following acute administration of MDMA. J Psychopharmacol. 2013;27:109-112.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebrospinal fluid (CSF) analysis and interpretation in neurocritical care for acute neurological conditions. Indian J Crit Care Med. 2019;23(Suppl 2):S115-S119.
- [CrossRef] [PubMed] [Google Scholar]
- Measurement of plasma tryptophan metabolites: clinical and experimental application for depression and stress states assessment. Melatonin Molecular Biology Clinical and Pharmaceutical Approaches. 2018;x:x.
- [CrossRef] [Google Scholar]
- Simultaneous determination of 5-hydroxyindoles and catechols by high-performanceliquid chromatography with fluorescence detection following derivatization with benzylamine and 1,2-diphenylethylenediamine. J Chromatogr A. 2003;1012:169-177.
- [CrossRef] [PubMed] [Google Scholar]
- Novel and sensitive high-performance liquid chromatographic method based on electrochemical coulometric array detection for simultaneous determination of catecholamines, kynurenine and indole derivatives of tryptophan. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;769:145-153.
- [CrossRef] [PubMed] [Google Scholar]
- Measurement of plasma 5-hydroxyindole acetic acid by liquid chromatography tandem mass spectrometry-comparison with HPLC methodology. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(78):695-699.
- [CrossRef] [PubMed] [Google Scholar]
- Development and validation of an ultra-high performance liquid chromatography-tandem mass-spectrometry (UHPLCMS/MS) method for the simultaneous determination of neurotransmitters in rat brain samples. J Neurosci Methods. 2011;198:187-194.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of urinary 5-hydroxy-3-indoleacetic acid by an automated HPLC method. Biomed Chromatogr. 1989;3:114-117.
- [CrossRef] [PubMed] [Google Scholar]
- Chapter 3: Investigating Proteins - Chemistry [Internet] [cited 2023 Feb 16]. Accessed August 18, 2023 at: https://wou.edu/chemistry/courses/online-chemistry-textbooks/ch450-and-ch451-biochemistry-defining-life-at-the-molecular-level/chapter-3-investigating-proteins/
- [Google Scholar]







