Translate this page into:
Depressed Monocytic Activity may be a Predictor for Sepsis
Address for correspondence: Dr. Purva Mathur, E-mail: purvamathur@yahoo.co.in
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Trauma is one of the leading causes of mortality worldwide with infections as important causes of death in such patients. Bacterial infections cause activation of monocytes with excessive synthesis of pro-inflammatory cytokines. Hence, this prospective study was conducted to assess the activity of monocytes in traumatized sepsis patients using flow cytometry and to assess if they have any prognostic potential.
Materials and Methods:
A total of 16 consecutive trauma patients with sepsis and having positive blood culture were enrolled, along with four healthy controls during the period of March 2013 to July 2013. Blood from septic patients were collected on the same day when blood culture was positive and on days 2 and 5 thereafter. Surface staining for monocytes with CD14 and intracellular staining for interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) was done and results were analyzed by flow cytometer. Procalcitonin (PCT) assay was done using MiniVidas. Complete clinical follow-up was done for the patients.
Results:
Of the 16 patients, four died due to infections by various microorganisms. Isolated abdominal trauma (25%) was the most common injury among the enrolled patients of sepsis. Levels of TNF-α were significantly decreased when stimulated with lipopolysaccharide in the fatal patients as compared to the healthy controls. Patients having sepsis who survived had an increased level of TNF-α during the follow-up periods.
Conclusion:
This study showed that activity of monocytes to produce TNF-α and IL-6 were reduced in severe sepsis. Early identification of such immune-paralysis can help in earlier intervention to salvage this vulnerable trauma population.
Keywords
Interleukin-6
monocytes
sepsis
trauma
tumor necrosis factor-alpha
INTRODUCTION
Accidents and trauma are one of the worlds’ most serious, but neglected health problems and is one of the leading causes of mortality in young adults. Globally, 26% of all deaths in the age group of 15–44 years in 2002 were due to injury.[123] In India, trauma is a major problem, due to a very high incidence of vehicular accidents, other accidents, injuries, crimes, and violence.[124]
Infections are one of the most important causes of mortality and morbidity in traumatized patients. Despite early effective treatment, such patients are susceptible to developing sepsis and multiple organ failure, which in turn are the major causes of death in these critically ill patients.[56789] Monocytes have been found to play a key role in immunological response to the bacterial infections.[1011] Systemic inflammation, as well as immune suppression, are thought to play a decisive role in the development of sepsis and multiple organ dysfunction syndromes. As the paradigm of sepsis pathogenesis has evolved over time, and our understanding of the different therapeutic approaches to sepsis developed, various biomarkers have been used for diagnosis of sepsis and monitoring of treatment. The diagnosis of sepsis is still a challenge till date due to the highly variable nature of its signs, symptoms, and unreliable performance of the available biomarkers.[12] Early diagnosis and stratification of the severity of sepsis will increase the possibility of appropriate and timely treatment.[13] The secretion of pro-inflammatory mediators results in an inadequately regulated inflammation. Pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) have been implicated as principal mediators of monocytic activity and probably major mediators involved in the pathogenesis of sepsis.[78914]
Most of the studies on the evaluation of the role of TNF-α and IL-6 have been done using plasma, which depicts extracellular pooled levels.[15] These assays may give an erroneous interpretation. Moreover, the cellular origin cannot be ascertained in these extracellular assays. Given the importance of sepsis diagnosis and the lacunae thereof, the present study was designed to assess the activity of monocytes with respect to production of TNF-α and IL-6 by flow cytometry in critically ill septic patients admitted to the Intensive Care Unit (ICU) of a level-1 trauma center and to correlate it with the patients’ outcomes.
MATERIALS AND METHODS
A prospective study was conducted from March 2013 to July 2013.
Patient selection
Patients enrolled in the present study were those whose blood cultures (one or more) had grown aerobic gram-negative pathogen/s and further fulfilled the criteria for clinical diagnosis of sepsis. The presence of sepsis was confirmed when at least two of the following four clinical signs of sepsis were present: (a) Temperature >38.9°C or <35.5°C; (b) respiratory rate <28 breaths/min or PaCO2 >32 torr; (c) heart rate >100 beats/min; (d) white blood cells >15,000 or <3500 cells/mm.[31617] Five milliliter blood samples were taken from these patients for assessment of monocytic activity by flow cytometry.
Healthy control
Healthy laboratory and hospital employees of both genders served as the control group. Four healthy controls were enrolled in the study.
Collection of blood and stimulation
Five milliliter of whole blood were collected from each patient and divided equally in heparinized (BD Vacutainer Lithium Heparin 75 USP units, BD Franklin Lakes NJ, USA) and plain vials from all patients on the day when their blood culture was positive (considered as day 0), and on days 2 and 5 thereafter as follow-ups to look for the patient's outcome and correlate it with monocytic activity. Serum was separated from the plain vial and stored at − 20°C for procalcitonin (PCT) assay whereas the heparinized blood was processed immediately for intracellular cytokine assay.
The heparinized blood was stimulated immediately as to prevent cell death over the period of time. For this, the whole blood was diluted in the ratio of 1:1 in RPMI 1640 medium (Thermo Scientific, South Logan, Utah, USA), and further divided equally into two tubes. One was challenged with 1 μg/mL lipopolysaccharide (LPS) (Escherichia coli, Serotype 055:B5, Sigma-Aldrich, St. Louis, MO, USA), incubated at 37°C in 5% carbon dioxide (CO2) with 1 μg/mL monensin (BD, San Jose, CA, USA) to inhibit protein secretion for 4 h. In parallel, the other tube, taken as basal (unstimulated) tube was also treated similarly, but without LPS to serve as a baseline comparison.[10]
Surface and intracellular staining of cells for flow cytometric analysis
After stimulation, 100 μl of blood was incubated with specific monoclonal antibodies for CD45 and CD14 antigen tagged with PerCP Cy5.5 and PE-Cy7 (BD, San Jose, CA, USA), respectively. After incubation, red blood cells were lysed with lysis buffer (BD, San Jose, CA, USA). The cells were then fixed and permeabilized by fixation and permeabilization buffer (BD, San Deigo, CA, USA), followed by incubation on ice in dark with intracellular specific antibodies for IL-6 and TNF-α tagged with Phycoerythrin and Allophycocyanin (BD, San Jose, CA, USA) respectively. The cells were then washed twice with wash buffer (BD, San Deigo, CA, USA), and the pellet was resuspended in 1% paraformaldehyde (Fischer-Scientific, Mumbai, India). The cells were then analyzed by flow cytometry.
Multiparameter flow cytometric analysis
Samples were then analyzed using multiparametric flow cytometer (BD FACS AriaIII, USA). Results were analyzed using BD FACS Diva software(BD biosciences) and gate was set around the monocyte population, which was strongly positive for CD14 on forward scatter on dot plots. TNF-α and IL-6 production from monocytes were then analyzed.
Also, PCT levels were assessed at all time frames from serum samples by the minividas system (BioMérieux, France). The test was performed according to the manufacturers’ instructions.
All the patients enrolled in the study were clinically followed-up till their final hospital outcome (discharge/death) and their clinical and laboratory finding were recorded daily, including the microbiological culture reports of all samples received in the laboratory. Detailed follow-ups of all blood culture reports of these patients were done along with daily assessment of clinical and microbiological findings. Details of surgical procedure performed, antimicrobial treatment and clinical as well as autopsy reports of fatal cases to find the cause of death were similarly noted.
Statistics
Results are recorded as mean ± standard deviation. Data were analyzed by the Mann–Whitney test for multiple comparisons. Differences were considered significant with P < 0.05.
The study was approved by the Institute's Ethical Committee.
RESULTS
Demographic profile of enrolled patients
A total of 16 critically ill trauma patients between the age group of 18 and 65 years, who were admitted to the trauma neurosurgery and surgery ICUs and whose blood cultures were positive for gram-negative bacilli in the microbiology laboratory were enrolled in the study. Among the enrolled patients, male (13, 81%) were more than the female (3, 19%) patients.
Clinical profile of enrolled patients
Of the 16 patients, 4 (25%) had isolated abdominal trauma, whereas 4 (25%) presented with both abdominal and chest trauma, 2 (13%) had isolated chest trauma, and the rest (6, 38%) suffered from multiple orthopedic injuries. Four patients (25%) had undergone exploratory laparotomy, whereas 4 (25%) other patients had to undergo tracheostomy, 2 (13%) underwent screw fixation of fractures and 1 (6%) each underwent splenectomy, corpectomy, and amputation respectively. Of the remaining, 3 (19%) had to undergo exploratory laparotomy in combination with either tracheostomy or splenectomy or gangrenous bowel resection.
Of these 16 patients, 12 (75%) were discharged after variable lengths of hospital stay (12–57 days). During their hospital stay, all of these 12 patients had one or more hospital-acquired infections. Septicemia developed in all the 12 patients with Acinetobacter baumannii, (7 [58%]) being the most commonly pathogen isolated from blood culture, followed equally by Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Candida albicans (2 [16%]) each and Klebsiella pneumonia 1 (8%).
The remaining four patients expired and the clinical and autopsy proven cause of death was cardiac arrest with septic shock. During their hospital stay, all four patients also developed septicemia and two of them had associated ventilator associated pneumonia. The microbiological data and other feature of these patients are detailed in Table 1.

Cytokines levels in patients who survived sepsis
-
TNF-α: Of the 16 patients, 12 (75%) patients survived the sepsis after variable lengths of hospital stay. In all the patients who survived, the TNF-α levels were found to increase gradually on consecutive follow-ups, but as compared with healthy controls, the levels were slightly decreased. However, in the last follow-up, the levels were slightly decreased [Table 2]
-
IL-6: This showed varied results in all the patients who survived, but statistically, the mean of IL-6 in all the patients after stimulation showed decreased level as compared to controls in every follow-up, although no uniform pattern was observed which makes the data statistically insignificant [Table 2]
-
PCT levels: Out of 12 patients who survived the sepsis, PCT level in eight patients were observed in normal range (0.5 ng/ml) and two each were observed to be positive (0.5–2 ng/ml) and highly positive (2–10 ng/ml).

Cytokines levels in the fatal patients
-
TNF-α level in patients with fatal outcome: Of the 16 patients, four patients expired due to suspected sepsis. In three patients the levels of TNF-α were found to be significantly decreased on stimulation with LPS as compared to healthy controls, from the day of blood culture positivity till the last follow-up on day 5th and found to be statistically significant. In the fourth patient, we have observed a rising level of TNF-α on each follow-ups, though it was significantly lower compared to the healthy controls [Figure 1 and Table 2]
-
IL-6 levels in patients with fatal outcome: Similarly, the levels of IL-6 post stimulation were also decreased in the same three patients mentioned above as compared to healthy controls but this difference was not found to be statistically significant. In the fourth patient, IL-6 levels increased with every follow-up [Table 3]
-
PCT levels in patients with fatal outcome: In the three patients with decreased levels of TNF-α and IL-6, PCT were found to be highly positive (2-10 ng/ml) in these patients. However, the PCT level in the fourth patient with fatal outcome, who had levels of TNF-α and raised IL-6 levels were found to be in normal range (0.5 ng/ml).
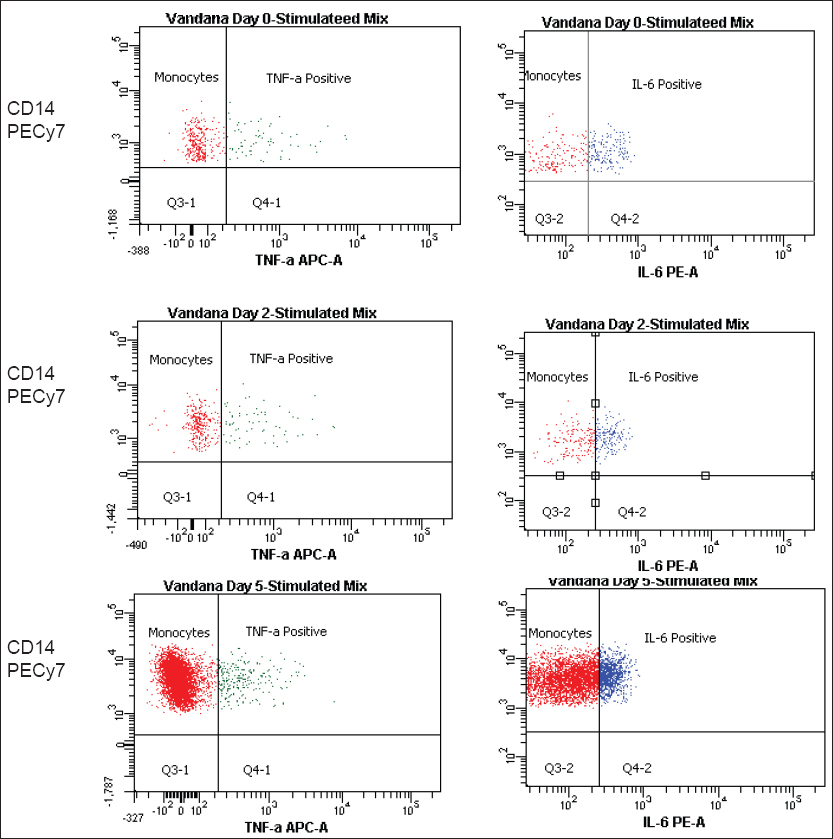
- The decreased level of tumor necrosis factor-alpha in fatal patients following sepsis is compared to healthy control. Levels of interleukin-6 in fatal cases were increased in first two follow-ups buts were significantly decreased in the last follow-up

A comparison between the cytokine levels of all the patient groups on different days are shown in Tables 2 and 3.
DISCUSSION
It is widely believed that bacterial endotoxin on entering the bloodstream triggers various alterations in the immune system, resulting in the release of various pro-inflammatory and inflammatory markers which may lead to a plethora of complications.[318] To study the kinetics of cytokines appearing in the blood of septic patients, detailed research is needed, which would help us find some novel methods of pharmacological interventions as well as new and early diagnostic markers during the initial inflammatory response. Ours is the first study from India monitoring the monocytic immune response in septic trauma patients. Most of the studies on cytokine production in septic patients have been done on plasma which in our view has two disadvantages.[19] First, studies on plasma would reflect the total production of cytokines rather than their cellular origin. Second, the short half-life of pro-inflammatory cytokines results in their rapid disappearance. This does not give the true picture regarding the cellular source of cytokines at a single cell level and the actual cytokine profile of posttrauma immune response.[20] To overcome this biological drawback, we used intracellular cytokine analysis utilizing the flow cytometric approach. Thus, we targeted the pro-inflammatory cytokines producing activity of monocytes by multiparametric flow cytometry, which represents a reliable method enabling highly selective immunomonitoring of specific cells in septic patients.
In septic patients who succumbed to trauma, we observed that the levels of TNF-α and IL-6 on stimulation with LPS were strongly blunted from day 1 of getting positive blood culture reports and continued to decline till further follow-ups (2nd and 5th day). Whereas, in those patients who survived the sepsis, after initial low levels of TNF-α, there was a gradual increase in the levels of TNF-α with each follow-up, as compared to the healthy controls. Hence, our data suggests a reduced capability of monocytes in whole blood from trauma patients in sepsis to release sufficient amounts of pro-inflammatory cytokines in response to the endotoxins encountered. All the patients had been started on antimicrobials based on their antibiotic sensitivity reports; however, those patients who showed continuously decreased levels of cytokines died and those whose cytokines levels increased ultimately survived the sepsis. This highlights the fact that effectively monocytic activity is compromised during sepsis, hence limiting the ability of the patient's immune system to adequately respond to invading microorganisms. Ertel et al. found a somewhat similar findings.[20]
The levels of IL-6 in the septic patients who survived gave variable results, hence as a sound conclusion cannot be drawn. This could also be due to the small sample size of our study. Many studies have described IL-6 as one of the principal mediators during endotoxemia.[14]
Procalcitonin levels in all except two septic patients who survived were below the cut-off levels (0.5 ng/ml), hence making TNF-α and IL-6 better markers for initial evaluation of sepsis.
Hence, our study suggests that both TNF α-and IL-6 would make a good marker for sepsis, though further studies are needed to substantiate it.
The strength of our study was that clinical follow-ups till the final outcome and autopsy-based confirmation of the cause of death. Trauma patients are a fairly uniform cohort, where underlying illness is few and most patients are in the middle age group. Our study is also not without its limitations. First, the study was done for a short duration and we need to increase the sample size. For better comparison, freshly admitted trauma patient's monocytic cytokine profile would have given a more holistic view of the immune response.
CONCLUSION
This study was done on trauma victims, who at the time of admission were not incubating any infections hence all the infection were hospital-acquired, which increases the morbidity and mortality. Thus, the findings of this study can be extrapolated to predicting early sepsis development and thus preventing the vulnerable young economically productive population of our country from severe complications after injury.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Identification and treatment of infections in multiply traumatized patients. Am J Med. 1985;79:68-76.
- [Google Scholar]
- World Health Organization Statistical Annex. World Report on Road Traffic Injury Prevention. Available from: http://www.who.int/violence_injury_prevention/publications/road_traffic/world_report/summary_en_rev.pdf(2011)
- [Google Scholar]
- Infections in traumatised patients: A growing medico-surgical concern. Indian J Med Microbiol. 2008;26:212-6.
- [Google Scholar]
- The “T” in trauma: The helper T-cell response and the role of immunomodulation in trauma and burn patients. J Trauma. 2007;63:1407-17.
- [Google Scholar]
- Suppression and recovery of LPS-stimulated monocyte activity after trauma is correlated with increasing injury severity: A prospective clinical study. J Trauma. 2009;66:1273-80.
- [Google Scholar]
- Depressed interleukin-12-producing activity by monocytes correlates with adverse clinical course and a shift toward Th2-type lymphocyte pattern in severely injured male trauma patients. Crit Care Med. 2003;31:1722-9.
- [Google Scholar]
- Systemic inflammatory response after extremity or truncal fracture operations. J Trauma. 2008;65:1379-84.
- [Google Scholar]
- Infectious complications in critically injured children. J Pediatr Surg. 2000;35:1174-8.
- [Google Scholar]
- Early down-regulation of the pro-inflammatory potential of monocytes is correlated to organ dysfunction in patients after severe multiple injury: A cohort study. Crit Care. 2009;13:R88.
- [Google Scholar]
- Clinical aspects: From systemic inflammation to ‘immunoparalysis’. Chem Immunol. 2000;74:162-77.
- [Google Scholar]
- Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg. 2000;135:291-5.
- [Google Scholar]
- The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187-96.
- [Google Scholar]
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864-74.
- [Google Scholar]
- What are the microbial components implicated in the pathogenesis of sepsis? Report on a symposium. Clin Infect Dis. 2000;31:851-8.
- [Google Scholar]
- Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215:356-62.
- [Google Scholar]
- Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood. 1995;85:1341-7.
- [Google Scholar]





