Translate this page into:
Determination of antibacterial activity and metabolite profile of Ruta graveolens against Streptococcus mutans and Streptococcus sobrinus
Address for correspondence: Dr. Hamzah Abdulrahman Salman, Department of Microbiology, J. J. College of Arts and Science, Pudukkottai, Tamil Nadu, India. E-mail: hamza.alayash@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
BACKGROUND:
Ruta graveolens is one of the most used phytomedicines. To date, there is no report of determining the bioactivity of R. graveolens against cariogenic causing bacteria (Streptococcus mutans and Streptococcus sobrinus).
OBJECTIVE:
The objective of the present study was to determine the antibacterial activity and metabolite profile of R. graveolens against S. mutans and S. sobrinus.
MATERIALS AND METHODS:
R. graveolens plant material was collected and processed in the month of February. The plant material was extracted by Soxhlet apparatus using methanol solvent. Two strains of S. mutans and two strains of S. sobrinus were isolated from dental caries-active participants and cultured on mitis salivarius-bacitracin agar. The antibacterial susceptibility testing of methanolic extract of R. graveolens was performed by disc diffusion method. The metabolite profile of the plant extract was determined using electrospray ionization-tandem mass spectrometry.
RESULTS:
The methanolic extract of R. graveolens showed a promising antibacterial activity against S. mutans and S. sobrinus. Two compounds named γ-fagarine and kokusaginine were identified from the methanolic extract of R. graveolens.
CONCLUSION:
The study concluded that R. graveolens contains significant antibacterial activity. However, further investigations are suggested to understand the anticaries properties of these pure compounds.
Keywords
γ-fagarine
anticariogenic
dental caries
electrospray ionization-tandem mass spectrometry
kokusaginine
mutans streptococci
Introduction
Dental caries is an irretrievable localized disease that consequences of the progressive tooth decay. Among oral microbiome, Streptococcus mutans and Streptococcus sobrinus are the key agents of causing dental caries, which are belonging to acid-producing bacterial group called mutans streptococci (MS).[12] However, a rare species of MS, Streptococcus dentapri, has been recently isolated from human dental caries.[3]
Due to the incremental cost of manufacturing of antibiotics and the rise of bacterial resistance to the presently available antimicrobial agents, new strategies of controlling the diseases such as the use of plant-derived extract were initiated.[45] Plants have been one of the essential sources of medicines from the start of human development. Green products continue to demonstrate high activity compounds with no side effects.[6]
The effect of polyphenols against MS have been studied in both in vitro and in vivo.[78] Extracts from unfermented green tea, cocoa, and the seeds of red grape, showed a high content of polyphenol, which has antibacterial activity against S. mutans.[9]
Ruta graveolens belongs to the family Rutaceae. It is known as Garden Rue in English, Satap in Hindi, Arvada in Tamil, Sudabugida in Kannada, Aruta in Malayalam, and Sadapaka in Telugu. R. graveolens reported to contain a large amount of secondary metabolites such as volatile oils, phenolic acids, flavonoids, and coumarins. Moreover, it is reported to consist pharmacological activities, i.e., antimicrobial, antiviral, anticancer, analgesic, free radical scavenging, antiplasmodial, anti-inflammatory, contraceptive effects, and antipyretic.[1011] R. graveolens is biologically valuable source of furoquinolone alkaloids and furanocoumarin. Linear furanocoumarins have been used as skin remedies, neurology, and pigmentation disorders.[1213] Furthermore, it has been reported that the methanolic extracts of R. graveolens possessed antioxidant activity.[14]
Despite the various pharmacological significances, the bioactivity of R. graveolens against S. mutans and S. sobrinus has not been studied. Therefore, this study was aimed to determine the antibacterial activity and metabolite profile of R. graveolens against S. mutans and S. sobrinus.
Materials and Methods
Bacterial isolates
The ethical approval of the present study was granted from the Institutional Ethics Committee of P.M.N.M Dental College, Bagalkot, Karnataka, India. Two clinical strains of each S. mutans and S. sobrinus bearing NCBI accession numbers KP975192, KP975193, KP975179, and KP975203, respectively, were previously isolated from dental plaques of four dental caries-active participants[15] were employed in this study. S. mutans ATCC 25175, S. mutans MTCC 497, and S. sobrinus ATCC 33478 were used as reference strains. All strains were cultured on mitis salivarius-bacitracin agar (HiMedia, India).[16]
Collection and processing of test plant material
R. graveolens was collected from UAS University, GKVK, Bangalore and authenticated by Dr. Vasundhara M, Professor in the Department of Horticulture, UAS University, GKVK, Bangalore, India. R. graveolens was collected and processed in the month of February 2015. The plant material was rinsed with sterile distilled water and kept in hot air oven to dry. The dried plant material was crushed using electrical blender and then stored in airtight bottle for further uses.
Extraction of plant material
The extraction of R. graveolens was performed using Soxhlet apparatus. Precisely, 40 g of dried R. graveolens was loaded with 600 ml of 100% v/v methanol (HPLC grade). The temperature of the Soxhlet apparatus was set at 25°C, and the extraction was carried out for 30 h.[15] The extract was filtered using Whatman paper no. 2. The filtrate was then concentrated by rotary vacuum evaporator and stored in a small screw cap bottle.
Preparation of antibacterial discs
The antibacterial susceptibility testing was performed by disc diffusion method as described by Zaidan et al.,[17] with a few modifications. Briefly, different concentrations (5, 10, 15, 20, 25, 30, 35, 40, 45, 50, and 100 mg) of plant extract were dissolved completely in 1 ml of 100% methanol solvent. Sterile disc papers (HiMedia, India) were aseptically soaked in sterile Petri dish containing the dissolved plant extract. Disc papers for the negative control were soaked in 100% methanol solvent. The impregnated discs were kept overnight in biosafety cabinet for complete methanol evaporation and immediately used for the sensitivity testing. The standard commercial antibiotic disc used for positive control was ampicillin 10 μg/disc (HiMedia, India).
Antibacterial susceptibility testing
All the test strains were cultured on brain heart infusion (BHI) agar[18] (HiMedia, India), and later one colony was inoculated into BHI broth. The culture broths were incubated at 37°C for 24 h. The bacterial inoculum was then adjusted to match the turbidity of 0.5 McFarland standards (HiMedia, India). The adjusted inoculum was aseptically swabbed on BHI agar using a sterile cotton swab. After 10 min, different concentrations of plant extract discs as well as negative control disc were aseptically placed on the swabbed agar medium. The positive control disc (ampicillin 10 μg/disc) was placed separately on the swabbed agar medium due its high zone of inhibition.[19] All the plates were anaerobically incubated for 24 h at 37°C. The zone of inhibition was measured in millimeter. The reliability and reproducibility of the results was confirmed by repeating the experiment in triplicate.
Electrospray ionization-Tandem mass spectrometry and data analysis
The methanolic extract of R. graveolens was centrifuged and the clear supernatant was directly infused into ion trap mass spectrometer using a single syringe infusion pump (KD Scientific, MA, USA) at a flow rate of 180 μl/h. The analytes were analyzed using HCT Ultra PTM Discovery (Bruker Daltonics, Germany) mass spectrometry operated in positive mode with nebulizer pressure of 10 psi, dry gas flow rate of 5 L/min, and temperature at 300oC. The most intense precursor ions were selected manually and subjected to fragmentation using helium gas as the collision gas for collision-induced dissociation experiments with MS/MS fragmentation amplitude of 1 V. The raw data were processed in data analysis software 4.1. To identify the metabolites, the precursor and fragment ions m/z was searched using METLIN database.
Statistical analysis
The means and standard deviation of the different concentration of the plant extract were determined. Shapiro–Wilk test was conducted to check the normality of the variables. Since the data fall under nonnormal distributions, Mann–Whitney U-test was applied to test the significant differences between the variables. All the parameters were carried out using R version 3.2.3 and validated by SPSS software version 21 (IBM Corporation, USA). The statistical analysis result with P ≤ 0.05 was considered statistically significant.
Results
The methanolic extract of R. graveolens exhibited antibacterial activity against S. mutans and S. sobrinus. The average of the zone of the inhibition of R. graveolens against S. mutans and S. sobrinus at different concentrations is presented in Table 1. However, the average of the zone of inhibition of the ampicillin (positive control) was 48 mm and 50 mm for S. mutans and S. sobrinus, respectively. The zones of inhibition of the methanolic extract of R. graveolens at different concentrations against S. mutans and S. sobrinus are presented in Figures 1 and 2, respectively. At concentrations between 15-25 mg/ml, the methanolic extract of R. graveolens showed significant differences (P < 0.05) between S. mutans and S. sobrinus.

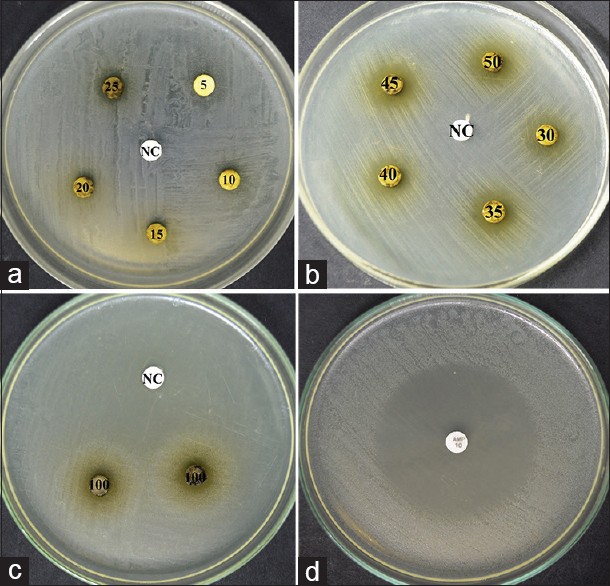
- Zones of inhibition of the methanolic extract of Ruta graveolens against Streptococcus mutans. (a) Concentration 5–25 mg/ml. (b) Concentration 30–50 mg/ml. (c) Concentration 100 mg/ml. (d) Positive control (ampicillin 10 μg/disc). NC: Negative control

- Zones of inhibition of the methanolic extract of Ruta graveolens against Streptococcus sobrinus. (a) Concentration 5–25 mg/ml. (b) Concentration 30–50 mg/ml. (c) Concentration 100 mg/ml. (d) Positive control (ampicillin 10 μg/disc). NC: Negative control
The metabolite profile of the methanolic extract of R. graveolens was obtained using electrospray ionization-tandem mass spectrometry (ESI-MSn). The direct injection of the sample showed the presence of a large group of compounds in the mass range of 200–350 m/z [Figure 3]. The MS2 of protonated precursor ions followed by MS3 of intense fragment ions were performed. The MS2 of a singly charged analyte at 229.72 m/z gave rise to intense fragment ions at 214.69 m/z indicating the loss of 14 Da (methyl group), and the further fragmentation of these ions indicated that the metabolite to be γ-fagarine [Figure 4]. Similarly, the MS2 of another singly charged analyte at 259.74 m/z revealed the fragmentation pattern similar to kokusaginine [Figure 5]. However, the identity of other analytes could not be found through the database search, thus indicating the presence of few uncharacterized metabolites.

- Electrospray ionization-tandem mass spectrometry spectra of methanolic extract of Ruta graveolens

- Electrospray ionization-tandem Ion trap mass spectrometry spectra of fragmentation of γ-fagarine from methanolic extract of Ruta graveolens. (a): MS2 of precursor ions at m/z 229.72. (b): MS3 of intense fragment ions at m/z 214.69
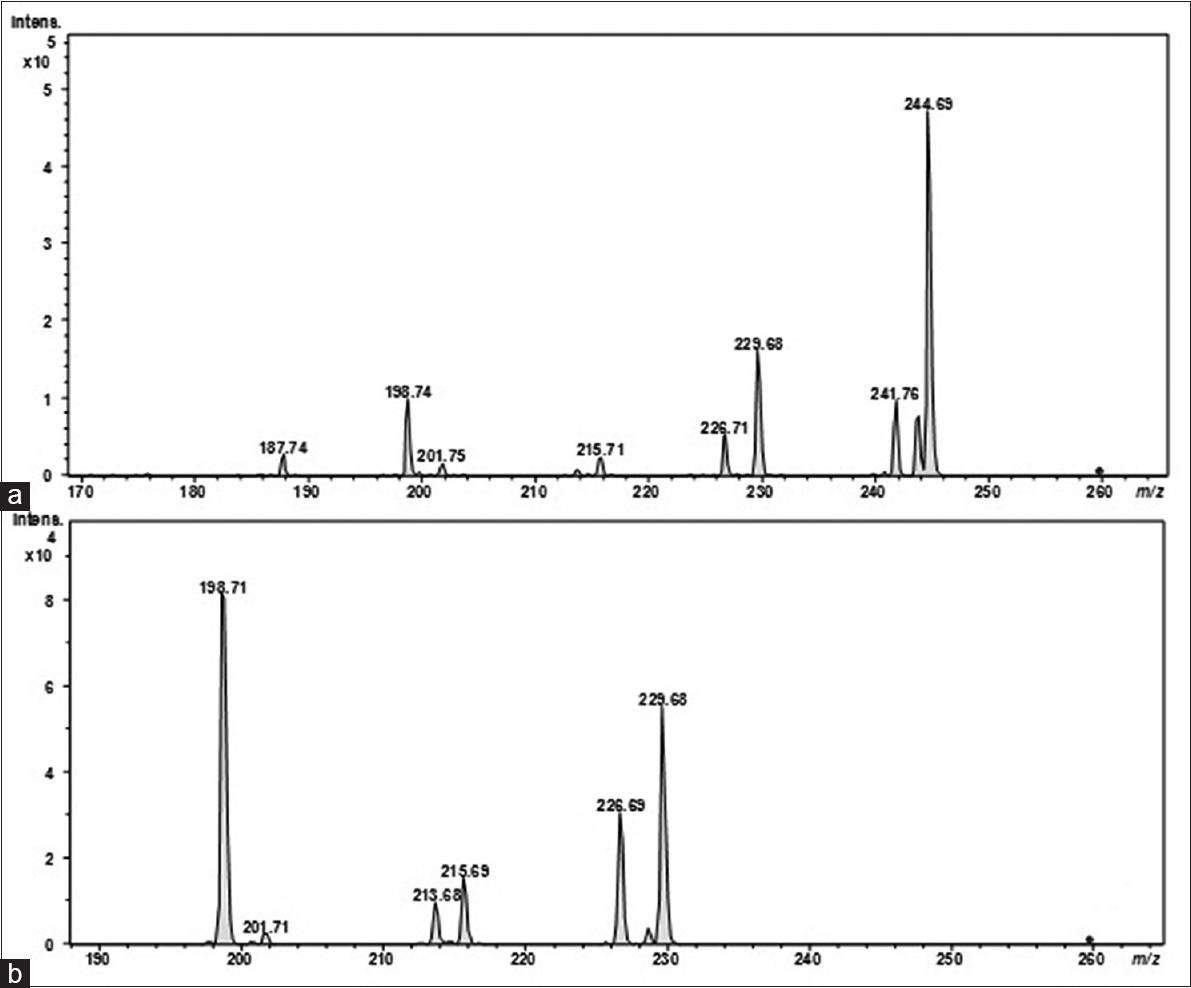
- Electrospray ionization-tandem Ion trap mass spectrometry spectra of fragmentation of kokusaginine from methanolic extract of Ruta graveolens. (a) MS2 of precursor ions at m/z 259.74. (b) MS3 of intense fragment ions at m/z 214.69
Discussion
In the present study, the prime cariogenic agents, S. mutans and S. sobrinus, were tested for their susceptibility by the methanolic extract of R. graveolens. The extract showed promising antibacterial activity against the tested organisms [Table 1]. After a broad literature search, this study is the first report to determine the antibacterial activity of R. graveolens against S. mutans and S. sobrinus.
At concentrations, 5 mg/ml and 10 mg/ml, no antibacterial activity of the plant extract has seen against S. mutans and S. sobrinus. While antibacterial activity was observed from concentration of 15–100 mg/ml. However, S. sobrinus was observed to be more susceptible than S. mutans at concentrations 15, 20, and 25 mg/ml. Furthermore, significant differences (P < 0.05) between S. mutans and S. sobrinus were found at the same concentrations [Table 1]. No statistically significant (P > 0.05) of different concentrations of the R. graveolens extract among the same species was observed. This is maybe due to the slightly increased in the zone of inhibition of each concentration [Figures 1 and 2].
The result demonstrated that, as the concentration of the plant extract increased from 40 to 100 mg/ml, the susceptibility of the tested organism remained the same. The reason for this, is unclear, might be attributed to the purity level of the extract or due to the diffusivity of the extract to the medium.
R. graveolens has been reported to contain alkaloids, phenolics, flavonoids, and flavanol compounds.[1020] In the current study, metabolite analysis of methanolic extract of R. graveolens was performed using ESI-MSn [Figure 3]. Among the possible metabolites, two compounds such as γ-fagarine [Figure 4] and kokusaginine [Figure 5] were detected and confirmed through fragmentation pattern. This finding is in accordance with the recent report.[21] Other analytes could not be found through the database search; therefore, the study revealed the presence of uncharacterized metabolites in the methanolic extract of R. graveolens. Hence, further studies are suggested to determine the undetected metabolites.
Based on earlier studies, γ-fagarine and kokusaginine were reported as antibacterial compounds.[2223] Furthermore, γ-fagarine and kokusaginine have various health benefits including antioxidant, cytotoxicity, antibacterial, antifungal, and other biological activities.[2425] Despite all these encouraging bioactivities of R. graveolens, limited information about the chemical components is available.[101421] This study could, however, be helpful in the assessment of the metabolites of R. graveolens.
Conclusion
Based on the evidence shown in the present study, methanolic extract of R. graveolens and certain of its compounds exert significant antibacterial activity against S. mutans and S. sobrinus, the main causative agents of dental caries. Further studies are recommended for in vitro testing of the pure compounds of γ-fagarine and kokusaginine against these organisms. In the future, the incorporation of such tested green products into mouthwash, chewing gum, toothpaste, and dental floss is a real opportunity in the way of controlling dental caries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Authors would like to thank the Nucleobase Life Science Research Laboratory, and Dr. Vasundhara M, Department of Horticulture, UAS University, GKVK, as well as Proteomics facility, Molecular Biophysics Unit, IISc, Bangalore for their valuable help.
References
- Isolation and typing of Streptococcus mutans and Streptococcus sobrinus from caries-active subjects. Contemp Clin Dent. 2017;8:587-93.
- [Google Scholar]
- First detection and characterization of Streptococcus dentapri from caries active subject. J Clin Diagn Res. 2017;11:DM01-3.
- [Google Scholar]
- Antibiotic resistance: A geopolitical issue. Clin Microbiol Infect. 2014;20:949-53.
- [Google Scholar]
- Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules. 2009;14:586-97.
- [Google Scholar]
- The antibacterial activity of plant extracts containing polyphenols against Streptococcus mutans. Caries Res. 2007;41:342-9.
- [Google Scholar]
- Ruta graveolens: Phytochemistry, pharmacology, and biotechnology. In: Jha S, ed. Transgenesis and Secondary Metabolism. Reference Series in Phytochemistry. Switzerland: Springer, Cham; 2017. p. :177-204.
- [Google Scholar]
- Algicidal and antifungal compounds from the roots of Ruta graveolens and synthesis of their analogs. Phytochemistry. 2005;66:2689-95.
- [Google Scholar]
- Accumulation of biologically active furanocoumarins in agitated cultures of Ruta graveolens L. and Ruta graveolens ssp. Divaricata (Tenore) gams. Pharmazie. 2005;60:623-6.
- [Google Scholar]
- Mode of action of psoralens, benzofurans, acridinons, and coumarins on the ionic currents in intact myelinated nerve fibres and its significance in demyelinating diseases. Gen Physiol Biophys. 1994;13:309-28.
- [Google Scholar]
- Phytochemical composition and antioxidant potential of Ruta graveolens L. in vitro culture lines. J Bot 2012 2012:685427.
- [Google Scholar]
- Antibacterial activity of Annona squamosa L. and Annona reticulata L. against clinical isolates of mutans streptococci the causative agents of dental caries. Asian J Pharm Clin Res. 2015;8:152-5.
- [Google Scholar]
- Isolation and characterization of the mutans streptococci from the dental plaques in Koreans. J Microbiol. 2007;45:246-55.
- [Google Scholar]
- In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Trop Biomed. 2005;22:165-70.
- [Google Scholar]
- Antimicrobial activity of few medicinal plants against clinically isolated human cariogenic pathogens – An in vitro study. ISRN Dent 2011 2011:541421.
- [Google Scholar]
- Identification and antibiogram profile of Streptococcus mutans and Streptococcus sobrinus from dental caries subjects. J Appl Pharm Sci. 2015;5:54-7.
- [Google Scholar]
- The chemical composition and the antibacterial properties of Ruta graveolens L. essential oil grown in Northern Jordan. Jordan J Biol Sci. 2015;8:139-43.
- [Google Scholar]
- Dereplication of phytochemicals in plants by LC-ESI-MS and ESI-MS n. TrAC Trends Anal Chem. 2012;33:46-54.
- [Google Scholar]
- Antibacterial activity and cytotoxicity of extractives from Ravenia spectabilis. Fitoterapia. 2004;75:510-3.
- [Google Scholar]
- Antimicrobial activity of the methanolic extract and compounds from Teclea afzelii (Rutaceae) S Afr J Bot. 2008;74:572-6.
- [Google Scholar]
- Photo-activated DNA binding and antimicrobial activities of furoquinoline and pyranoquinolone alkaloids from Rutaceae. Planta Med. 2004;70:531-5.
- [Google Scholar]
- Cytotoxic activity and cell cycle analysis of quinoline alkaloids isolated from Haplophyllum canaliculatum Boiss. Planta Med. 2009;75:1509-16.
- [Google Scholar]





