Translate this page into:
Distribution of various histopathological types of ovarian tumors: A study of 212 cases from a tertiary care center of Eastern Uttar Pradesh
Address for correspondence: Dr. Shashikant C. U. Patne, Department of Pathology, Homi Bhabha Cancer Hospital, (A Unit of Tata Memorial Centre, Mumbai), Varanasi - 221 002, Uttar Pradesh, India. E-mail: scupatne@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
BACKGROUND:
Ovarian tumors are one of the leading cancers in females with variable pathological types. This study describes the distribution, clinical and pathological details of various histopathological types of ovarian tumors in a tertiary care hospital in North India.
MATERIALS AND METHODS:
A retrospective data of 3 years were collected for ovarian tumors submitted to the pathology department of a tertiary care hospital. Data were classified according to the latest World Health Organization (WHO) Classification into epithelial tumors, germ cell tumors, sex cord–stromal tumors, and others.
RESULTS:
A total of 212 cases of ovarian tumors were studied, 186 were unilateral and 26 were bilateral. Resection specimen, part of specimen, and block review formed 80.2%, 15.1%, 4.7%, respectively. Epithelial tumors formed the majority in 71.7% of cases followed by germ cell tumors (22.2%), sex cord–stromal tumors (3.8%) and others (2.3%). Maximum number of cases in the respective groups occurred in the age groups 31–40, 21–30, 51–60, and 41–50 years, respectively. Overall, benign tumors were 63.7%, malignant tumors were 31.1%, and borderline were 5.2%. The most common histopathological type of benign and malignant tumor was benign serous cystadenoma (18.8%) and serous carcinoma (9.9%), respectively.
CONCLUSION:
In the present study, ovarian tumors were classified according to the WHO classification, epithelial and germ cell tumors were the major types of ovarian tumors. Benign epithelial tumor formed the majority with 46.2% cases. Serous cystadenoma and mature cystic teratoma were the predominant type of epithelial and germ cell tumors, respectively.
Keywords
Gynecologic malignancy
tumor markers
World Health Organization classification
Introduction
Ovarian malignancies are one of the common malignancies affecting women.[1] Worldwide, it is sixth most common cancer overall and second most common among gynecological cancers. It consists of 4% of all female cancers[2] and 23% of all gynecological cancer worldwide.[3]
In India, during 2004–2005, ovarian cancer ranked 3rd/4th among all cancers in women. Age-standardized incidence rate for ovarian cancers in India varies with region and ranges from 0.9 to 8.4 per 100,000 person-years with its highest incidence in Delhi and Pune.[1]
The prognosis of ovarian tumors depends on tumor stage, specific histological type and grading. Staging of ovarian tumors is done by TNM system, established by the American Joint Committee on Cancer, and also by the International Federation of Gynecology and Obstetrics staging system. Histological typing and grading are done mainly by the World Health Organization (WHO) classification of ovarian tumors. The identification of various histological patterns is important for predicting tumor behavior to decide further management of patients.[4]
The WHO classification is a comprehensive classification that takes into account various ovarian tumors. Defining criteria for borderline tumors are evolving. We have adapted the latest WHO classification, which is revised in 2014 by Robert Kurman and coauthors. In this classification, the demarcation between benign and borderline epithelial tumors has been revised. Serous tumors with more than 10% borderline architecture are classified as serous borderline tumors (SBT). If borderline architecture is <10%, it is classified as “serous cystadenoma with focal epithelial proliferation.” However, the diagnostic criteria for SBT remain the same. Microinvasion criteria have been modified in SBT. Only small clusters (<5 mm) with cytology similar to epithelium in SBT surface and surrounded by retraction spaces are defined as microinvasive foci. Whereas solid nests or cribriform glands cytologically resembling low-grade serous carcinoma (LGSC), even if <5 mm, are now categorized as LGSC regardless of their size.[5] Molecular and genetic studies of SBT are similar to that of LGSC. SBT-micropapillary variant is now a subtype of SBT.[5] In latest WHO classification, peritoneal implants have been described as invasive and noninvasive implants. Only noninvasive implants can be associated with borderline serous tumors. Any invasive implants are now a feature of LGSC.[5]
In borderline mucinous tumor, subcategorization into intestinal and endocervical type has been removed. The entity “endocervical type of mucinous borderline tumors” has been termed as “seromucinous tumor.” Moreover, intestinal type of mucinous borderline tumors is known as mucinous borderline tumor/atypical proliferating mucinous tumor.[5]
Previously described term “tumor with low malignant potential” is no longer used for borderline tumors.[5]
This study describes the relevant clinicopathological details and distribution of various histopathological types of ovarian tumors in a tertiary care hospital setting.
Materials and Methods
Data of all ovarian specimen and review blocks submitted to pathology department in 3 years (January 2015–December 2017) were retrieved retrospectively, and clinical and histological features were reviewed from the archival material in the pathology department. Cases diagnosed as ovarian tumors were included in the study. Data were classified according to the latest WHO Classification (2014) into epithelial tumors, germ cell tumors, sex cord–stromal tumors and others. Cases were distributed according to laterality, consistency, size, age, and histogenesis.
Observations
A total of 214 cases of ovarian tumors were collected. Two cases were excluded from the study due to the uncertainty of the diagnosis. Rest of 212 were distributed according to laterality, consistency, size, age, and histogenesis. Out of 212, 170 (80.2%) were resected specimens, 32 (15.1%) were parts of tumor and 10 (4.7%) were received as block review. Data were classified according to the latest WHO Classification into epithelial tumors, germ cell tumors, sex cord–stromal tumors and others (including metastatic carcinoma and non-Hodgkin lymphoma). Total number of cases in respective groups was 152 (71.7%), 47 (22.2%), 8 (3.8%), and 5 (2.3%). These cases were further subclassified according to latest WHO classification [Table 1 and Figures 1–9].
| Type of tumor | Number of cases (%) | ||
|---|---|---|---|
| Epithelial tumor | |||
| Serous tumor | Benign | Serous cystadenoma | 40 (18.9) |
| Borderline | Serous borderline tumor | 2 (0.9) | |
| Malignant | Low grade/high grade serous carcinoma | 21 (9.9) | |
| Mucinous tumor | Benign | Mucinous cystadenoma | 31 (14.6) |
| Borderline | Mucinous borderline tumors | 9 (4.2) | |
| Malignant | Mucinous carcinoma | 13 (6.1) | |
| Endometrioid tumor | Benign | Endometriotic cyst | 23 (10.8) |
| Malignant | Endometrioid carcinoma | 4 (1.9) | |
| Brenner tumor | Malignant | Malignant Brenner tumor | 3 (1.4) |
| Seromucinous tumor | Benign | Seromucinous cystadenoma | 4 (1.9) |
| Undifferentiated carcinoma | - | - | 2 (0.9) |
| Sex cord-stromal tumors | |||
| Pure stromal tumor | Fibroma | 3 (1.4) | |
| Thecoma | 1 (0.5) | ||
| Sclerosing stromal tumor | 1 (0.5) | ||
| Pure sex cord tumors | Adult/juvenile granulosa cell tumor | 3 (1.4) | |
| Germ cell tumors | |||
| Dysgerminoma | - | 1 (0.5) | |
| Yolk sac tumors | - | 1 (0.5) | |
| Embryonal carcinoma | - | 1 (0.5) | |
| Mature teratoma | - | 28 (13.2) | |
| Immature teratoma | - | 7 (3.3) | |
| Mixed germ cell tumor | - | 5 (2.4) | |
| Monodermal teratoma and somatic type tumors arising from dermoid cyst | |||
| Struma ovarii | Benign | 3 (1.4) | |
| Carcinoid | - | 1 (0.5) | |
| Others | |||
| Lymphoid tumor | Lymphoma | 1 (0.5) | |
| Secondary tumors | - | 4 (1.9) | |
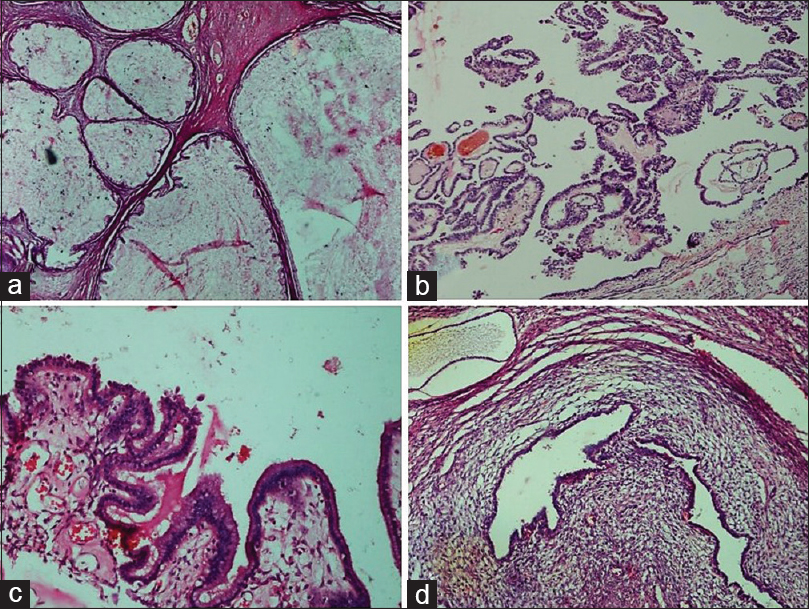
- Benign epithelial tumors. (a) Mucinous cystadenoma. Multilocular cyst filled with mucin and lined by simple columnar mucinous epithelium (H and E, ×4). (b) Serous cystadenoma with focal epithelial proliferation. Epithelial proliferation, stratification, and tufting is present in <10% of the total otherwise benign tumor (H and E, ×10). (c) Seromucinous cystadenoma. Cyst partly lined by benign serous epithelium and partly by benign endocervical-type mucinous epithelium (H and E, ×20). (d) Endometriotic cyst. Cyst shows the presence of benign endometrial glands surrounded by endometrial stroma (H and E, ×10)
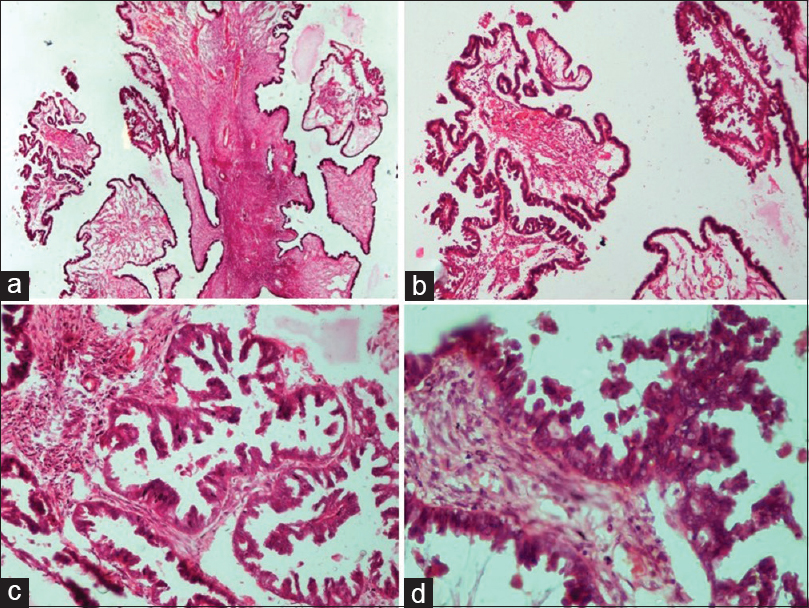
- Borderline epithelial tumors: Serous borderline tumor. (a) Irregular hierarchical branching papillae with detached tufts of epithelial cells (H and E, ×4). (b) Higher power view of the same (H and E, ×10). (c) Papillary branching and bridging with epithelial budding and detached tumor cells into the lumen (H and E, ×20). (d) Papillae lined by epithelium showing cytological atypia, cellular stratification and tufting (H and E, ×40)
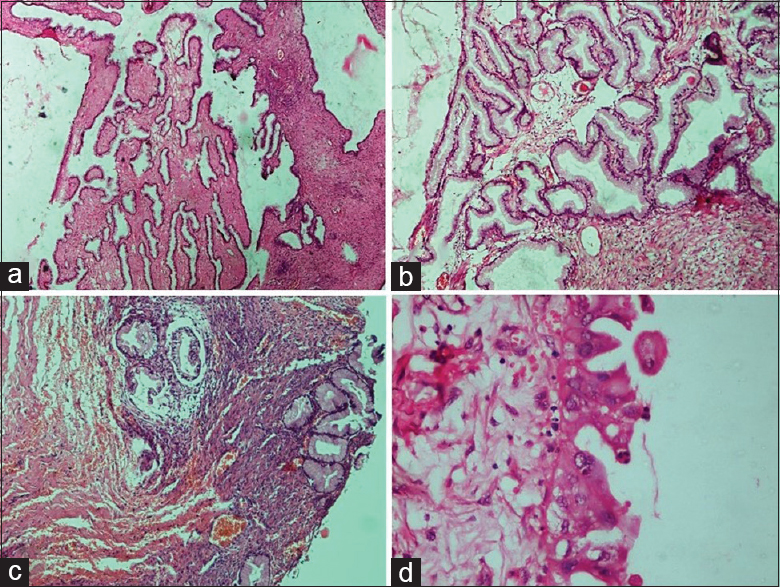
- Borderline epithelial tumors: Mucinous borderline tumor. (a) Extensive mucinous epithelial proliferation than seen in benign mucinous tumor with no destructive stromal invasion (H and E, ×4). (b) Complex glands and papillae lined by mucinous epithelium (H and E, ×10). (c) Focal area of microinvasion surrounded by a clear space (H and E, ×4). (d) Mucinous epithelium with round to oval vesicular nuclei and moderate nuclear atypia (H and E, ×40)
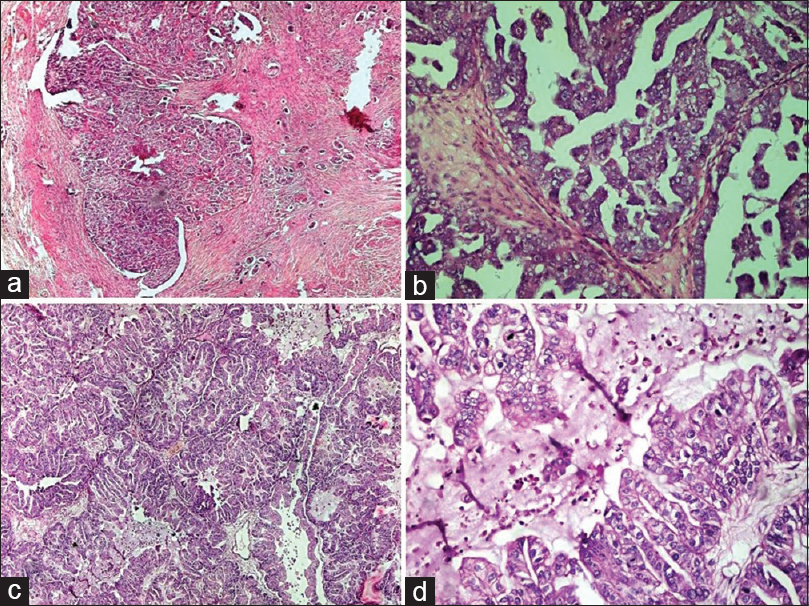
- Malignant epithelial tumors: (a and b) High-grade serous carcinoma. (a) The solid mass of tumor cells with slit-like spaces. Stromal infiltration is evident (H and E, ×4). (b) The tumor cells are forming papillary arrangement. Cells show a marked degree of cytological atypia and pleomorphism (H and E, ×20). (c and d) Mucinous carcinoma. (c) Solid growth with marked glandular crowding and very little intervening stroma. The lumen of the glands are filled with mucin (H and E, ×4). (d) Glands are lined by malignant mucinous epithelium showing marked nuclear atypia and intracytoplasmic mucin (H and E, ×20)
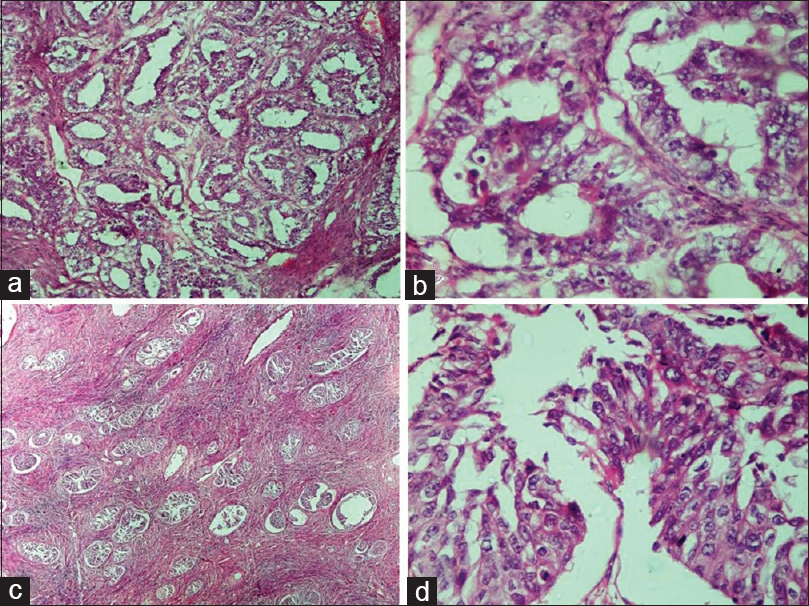
- Malignant epithelial tumors. (a) Endometrioid carcinoma. Back to back arrangement of the glands with stromal invasion (H and E, ×10). (b) Endometrioid carcinoma. Glands are lined by columnar tumor cells with atypia resembling endometrioid carcinoma of uterus (H and E, ×40). (c) Malignant Brenner tumor. Transitional cell nests of variable size are present with surrounding desmoplastic stroma (H and E, ×4). (d) Malignant Brenner tumor. Transitional epithelium showing moderate nuclear atypia and occasional mitosis (H and E, ×40)
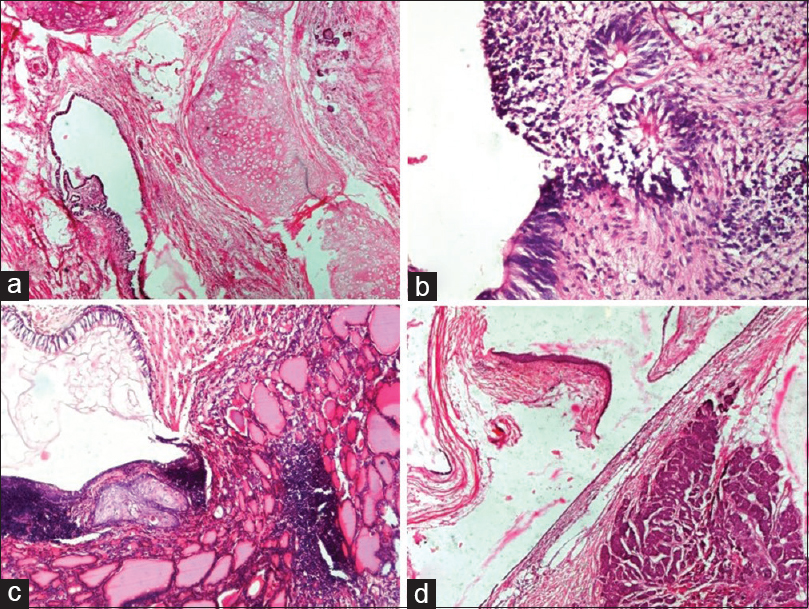
- Germ cell tumors: (a) Mature teratoma. Cyst lined by pseudostratified ciliated columnar epithelium with the presence of cartilage (H and E, ×4). (b) Immature teratoma. Cyst lined by immature neuroepithelium and presence of primitive neuroectodermal rosettes (H and E, ×20). (c). Struma ovarii. Cyst lined by respiratory epithelium and presence of benign thyroid follicles filled with colloid and focal cartilage (H and E, ×10). (d) Carcinoid tumor with mature teratoma. Cyst lined by squamous epithelium and another part of cyst shows carcinoid tumor composed of nests of neuroendocrine cells in stroma (H and E, ×4)
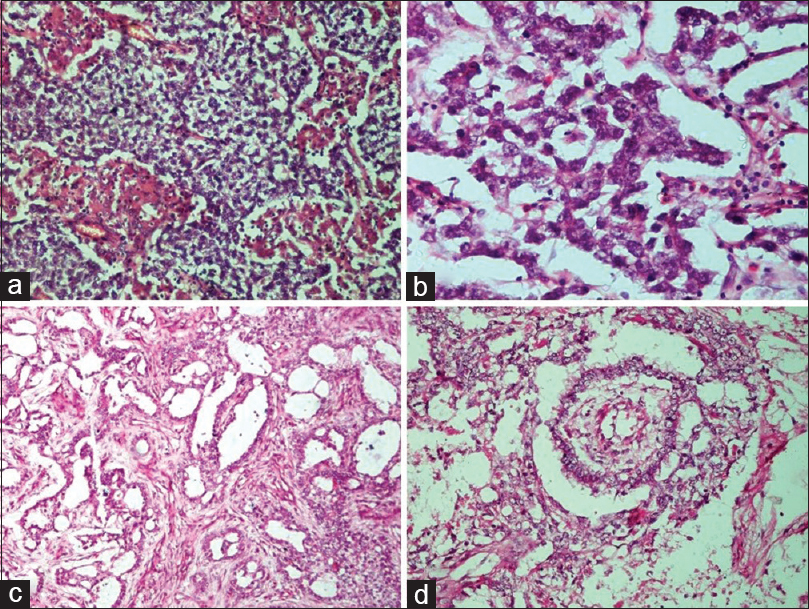
- Germ cell tumors. (a) Dysgerminoma. Sheets and lobules of tumor cells intersected by fibrous septa that is infiltrated by lymphocytes (H and E, ×20). (b) Dysgerminoma. The tumor cells have round vesicular nuclei with prominent nucleoli, abundant cytoplasm with well-defined cell membranes (H and E, ×40). (c) Yolk sac tumor. The reticular pattern of tumor cells in loose stroma (H and E, ×10). (d) Yolk sac tumor. Schiller–Duval body showing fibrovascular core or capillary in center surrounded by tumor cells inside a space lined by tumor cells (H and E, ×20)
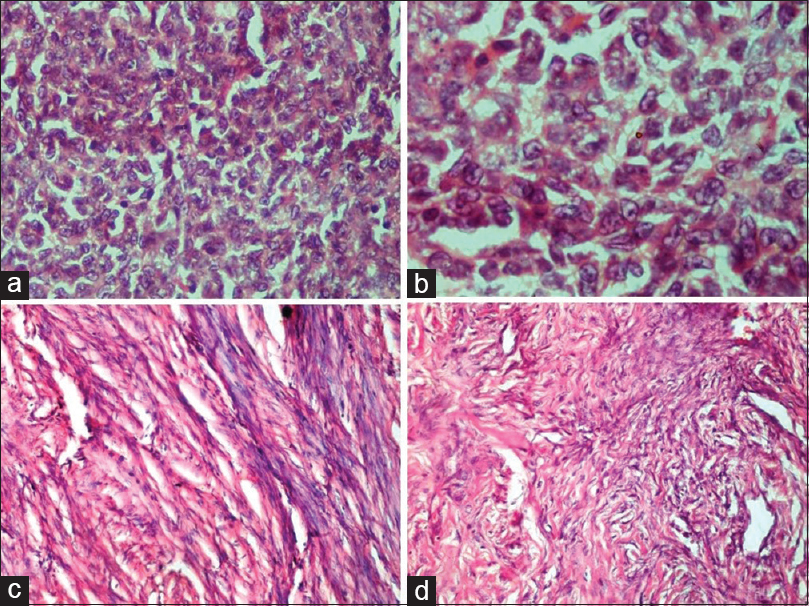
- Sex cord–stromal tumors. (a and b) Granulosa cell tumor. (a) Sheets of tumor cells with scant cytoplasm, absence of nuclear atypia and occasional nuclear grooves (H and E, ×40). (b) Nuclear grooving in prominent in some of the tumor cells (H and E, ×100). (c) Fibroma. Cells with spindled to ovoid-shaped bland nuclei and eosinophilic cytoplasm arranged in intersecting bundles (H and E, ×20). (d) Sclerosing stromal tumor. The tumor disposed in lobules with interspersed thin-walled blood vessels show round and spindled cells with occasional vacuolated cells in collagenous stroma (H and E, ×20)
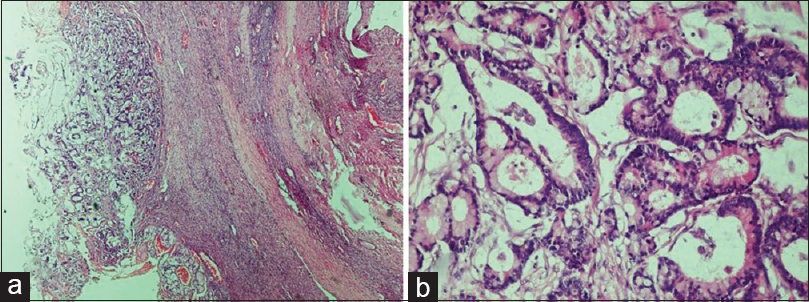
- Metastatic carcinoma. (a) Presence of malignant glands compressing the ovarian stroma (H and E, ×4). (b) The glands are lined by columnar epithelium showing nuclear atypia. The presence of Paneth cell indicates the origin of primary from gastrointestinal tract (H and E, ×20)
Laterality
Of 212, 186 (87.7%) were unilateral and 26 (12.3%) were bilateral. Out of 152 epithelial tumors, 132 (86.8%) were unilateral and 20 (13.2%) were bilateral. Out of 47 germ cell tumors, 41 (87.2%) were unilateral, and 6 (12.8%) were bilateral. Sex cord–stromal and metastatic tumors were only unilateral.
Consistency
Tumor was cystic in 113 (56%) cases, solid-cystic in 65 (32.1%) and solid in 24 (11.9%) cases. Rest 10 cases were block review for which gross details were not mentioned. Consistency in epithelial tumors was cystic in 93 (63.7%), solid-cystic in 41 (28.1%), and solid in 12 (8.2%). Rest six cases were received as block review. Amongst germ cell tumors, 20 (43.5%) were cystic, 21 (45.6%) were solid-cystic, 5 (10.9%) were solid and 1 was block review. Of sex cord–stromal tumors, 2 (33.3%) were cystic + solid, 4 (66.7%) were solid and 2 were block review. Amongst other category tumors, 1 (25%) was solid-cystic, 3 (75%) were solid and 1 was block review.
Size
Size-wise distribution of resected specimens was done. Of total 170 resected specimens, 25 (14.7%) were in size range of up to 5 cm, 71 (41.8%) were in range of 5–10 cm, 41 (24.1%) in range of 10–15 cm, 18 (10.6%) in 15–20 cm, 10 (5.9%) in 20–25 cm, 5 (2.9%) in 25–30 cm size range. Mean size of tumor in epithelial tumor, germ cell tumor, sex cord–stromal tumor and others category was 11.2 ± 6.9 cm, 11.5 ± 4.8 cm, 8.5 ± 2.8 cm, 9.2 ± 5.3 cm with the size range of 1–30 cm, 4–24 cm, 4–12 cm, and 5.4–13 in cm, respectively.
Age
Of total 212 cases, there were 7 (3.3%) cases in age range of up to 10 years, 24 (11.3%) in range of 11–20, 51 (24.1%) in range of 21–30, 51 (24.1%) in range of 31–40, 44 (20.8%) cases in range of 41–50, 30 (14.1%) cases in range of 51–60, 2 (0.9%) cases in range of 61–70, 2 (0.9%) in range of 71–80, and 1 (0.5%) in range of 81–90 years.
Mean age of cases in epithelial tumor, germ cell tumor, sex cord–stromal tumor and others category was 38.1 ± 12.6, 28.4 ± 17.7, 39.3 ± 18.6, and 45.2 ± 7.8 years with the age range of 15–76 years, 6 days–84 years, 15–60 years, and 38–58 years, respectively. Each category is distributed as per developmental life stages or age groups [Tables 2 and 3].
| Age range (years) | Epithelial tumor (%) | Germ cell tumor (%) | Sex cord-stromal tumor (%) | Others (%) | Total (%) |
|---|---|---|---|---|---|
| Childhood + adolescence (0-18) | 3 (17.6) | 13 (76.5) | 1 (5.9) | - | 17 (100) |
| Adult (18-40) | 87 (75.0) | 24 (20.7) | 3 (2.6) | 2 (1.7) | 116 (100) |
| Middle age (40-60) | 58 (78.3) | 9 (12.2) | 4 (5.4) | 3 (4.1) | 74 (100) |
| Old age (60+) | 4 (80) | 1 (20) | - | - | 5 (100) |
| Total | 152 (71.6) | 47 (22.1) | 8 (3.7) | 5 (2.3) | 212 (100) |
| Age range (years) | Epithelial tumor (%) | Germ cell tumor (%) | Sex cord-stromal tumor (%) | Others (%) | Total |
|---|---|---|---|---|---|
| Childhood+adolescence (0-18) | 3 (2.0) | 13 (27.7) | 1 (12.5) | - | 17 |
| Adult (18-40) | 87 (57.2) | 24 (51.0) | 3 (37.5) | 2 (40) | 116 |
| Middle age (40-60) | 58 (38.2) | 9 (19.2) | 4 (50) | 3 (60) | 74 |
| Old age (60+) | 4 (2.6) | 1 (2.1) | - | - | 5 |
| Total | 152 (100) | 47 (100) | 8 (100) | 5 (100) | 212 |
Histogenesis/nature
Overall, benign tumors were 135 (63.7%), malignant tumors were 66 (31.1%), and borderline were 11 (5.2%). Of 135 benign tumors, 127 (94.1%) were unilateral and 8 (5.9%) were bilateral. Of 66 malignant tumors, 52 (78.8%) were unilateral and 14 (21.1%) were bilateral. Of 11 borderline tumors, 7 (63.6%) were unilateral and 4 (36.4%) were bilateral.
Number of benign, malignant, and borderline tumors in each histological category is shown in Table 4. Total number of malignant cases in each category is described in Table 5.
| Type of tumor | Benign (%) | Malignant (%) | Borderline (%) |
|---|---|---|---|
| Epithelial tumor | 98 (72.6) | 43 (65.1) | 11 (100) |
| Germ cell tumor | 32 (23.7) | 15 (22.7) | - |
| Sex cord - stromal tumor | 5 (3.7) | 3 (4.5) | - |
| Others | - | 5 (7.6) | - |
| Total | 135 (100) | 66 (100) | 11 (100) |
| Type of tumor | Number of cases (%) |
|---|---|
| Epithelial tumors | |
| Serous carcinoma | 21 (31.8) |
| Mucinous carcinoma | 13 (19.7) |
| Endometrioid carcinoma | 4 (6.1) |
| Malignant Brenner tumor | 3 (4.5) |
| Undifferentiated carcinoma | 2 (3.0) |
| Germ cell tumors | |
| Immature teratoma | 7 (10.7) |
| Mixed germ cell tumor | 5 (7.6) |
| Dysgerminoma | 1 (1.5) |
| Yolk sac tumor | 1 (1.5) |
| Embryonal carcinoma | 1 (1.5) |
| Sex cord - stromal tumor | |
| Granulosa cell tumor | 3 (4.5) |
| Others | |
| Metastatic carcinoma and NHL | 5 (7.6) |
| Total | 66 (100) |
NHL = Non-Hodgkin lymphoma
Discussion
In this study of 212 ovarian tumors, epithelial tumors formed the majority in 71.7% of cases followed by germ cell tumors (22.2%). Similar observation were made by various other studies.[6789101112]
The most common tumor overall was benign serous cystadenoma (18.9%) followed by benign mucinous cystadenoma (14.6%) and mature teratoma (13.2%). Studies done by Garg et al., Patil et al., and Modepalli and Venugopal had similar findings.[6713] However, in Mankar and Jain[11] mucinous cystadenoma (32.69%) was the most common tumor.
In the present study, the most common malignant tumor was serous carcinoma. The most common borderline tumor was borderline mucinous tumor (4.2%). This finding are in concordance with those of a study carried out by Kanpurwala et al., Garg et al., and Modepalli and Venugopal.[6913] However, in Mondal et al.,[12] borderline serous tumor was the most common borderline tumor.
In the present study, 186 (87.7%) cases were unilateral and 26 (12.3%) were bilateral. The findings were almost similar to the studies conducted by Kanpurwala et al., and Modepalli and Venugopal.[913] Whereas in Garg et al.,[6] the frequency of bilateral cases was comparatively less (4.7%) which could be due to the inclusion of relatively lesser number of cases (n = 85) in their study.
In the present study, majority of tumors were unilateral in both benign (94.1%) and malignant tumors (78.8%). Similar observations were made by Manoja et al., and Kuladeepa et al.[1415] However, bilaterality was common in malignant tumors (53.8% of bilateral cases). Out of total 14 bilateral cases, serous carcinoma was most common (9 cases, 64.3%), 2 (14.3%) were mucinous carcinoma, 2 (14.3%) were mixed germ cell tumors and 1 (7.1%) was endometrioid carcinoma.
In our study, maximum number (41.8%) of cases were in size range of 5–10 cm which is similar to the study conducted by Manoja et al.[14]
In the present study, majority of ovarian tumors were cystic (56%), followed by mixed solid-cystic (32.1%) and least common was solid (11.9%). The study done by Garg et al.[6] and Kanpurwala et al.[9] had a similar observation of cystic as a most common consistency. However, the second common consistency was solid in Kanpurwala et al.[9] possibly because of inclusion of more number of malignant tumors (35.66%) which are commonly solid in consistency.
In the present study, epithelial tumors were mostly cystic, however in germ cell tumors, consistency was mostly solid-cystic and in sex cord–stromal tumors, they were mostly solid. This finding has not been observed in other similar studies.
Overall ovarian tumors were found in the age range of 11–76 years in the studies conducted by Garg et al., Kanpurwala et al., Mankar and Jain, and Modepalli and Venugopal[691113] However, in Sanjeev et al.,[8] the lowest age for ovarian tumors was 2 years. In our study, the lowest age for ovarian tumor was 6 days in two cases of germ cell tumor; one was mature teratoma and another was immature teratoma. To the best of our knowledge, this is the first study reporting a germ cell tumor at such a young age. In literature, youngest age of mature teratoma was reported in a 2-year-old child[16] and immature teratoma in a 4-year-old.[17] Maximum age of ovarian tumors was 84 years in our study which corroborated well with the studies done by Patil et al. and Sanjeev et al.[78] Maximum number (48.2%) of cases overall were in the age range of 21–40 years. Similar observations were made by Mankar and Jain.[11]
In childhood and adolescence, the most common tumor was germ cell tumor. In all other age groups, epithelial tumors were more common. However, the common age group for epithelial and germ cell tumor was adult age (18–40 years) and sex cord–stromal tumors and metastatic carcinoma were more common in middle age (40–60 years).
In the present study, 135 (63.7%) tumors were benign, 66 (31.1%) were malignant and 11 (5.2%) were borderline. Similar observations were made by Mankar and Jain and Sanjeev et al.[811]
In this study, most common malignant tumor overall was serous carcinoma (9.9% overall and 31.8% of malignant tumors) followed by mucinous carcinoma (6.1% overall and 19.7% of malignant tumors). This finding correlated well with the studies of various authors.[7891011121314] Interestingly, in a study by Garg et al. and Kanpurwala et al., the second common malignant tumor was granulosa cell tumor.[69]
Conclusion
This study describes the distribution, clinical and pathological details of ovarian tumors in a tertiary care hospital in North India. Following WHO classification, epithelial and germ cell tumors were the predominant type of ovarian tumors in our study. Benign epithelial tumor formed the majority with 46.2% cases with benign serous cystadenoma being the most common. Mature cystic teratoma was the main type of germ cell tumor. Almost one-third of the ovarian tumors were malignant with most common being serous carcinoma. More than half of the bilateral cases were malignant. Among borderline tumors, mucinous tumors were more common than serous. In childhood and adolescence, the most common tumor was germ cell tumor. In this study, we report two cases of germ cell tumors at 6 days of age, and this is the first study reporting germ cell tumors in such a young age.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Changing trends in incidence of ovarian cancer – The Indian scenario. Asian Pac J Cancer Prev. 2009;10:1025-30.
- [Google Scholar]
- Worldwide burden of gynaecological cancer: The size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006;20:207-25.
- [Google Scholar]
- A histopathological overview of ovarian lesions in Benin City, Nigeria: How common are the functional cysts? Int J Med Public Health. 2014;4:265-8.
- [Google Scholar]
- Pathology and classification of ovarian tumours. North American Association of Central Cancer Registries. Cancer Suppl. 2003;97:2631-42.
- [Google Scholar]
- Ovarian borderline tumors in the 2014 WHO classification: Evolving concepts and diagnostic criteria. Virchows Arch. 2017;470:125-42.
- [Google Scholar]
- Study of histomorphological spectrum of ovarian tumours. Int J Med Health Res. 2017;3:12-20.
- [Google Scholar]
- Histomorphological study of ovarian tumours at a tertiary care centre. Ann Pathol Lab Med. 2017;4:A638-45.
- [Google Scholar]
- A study of clinicomorphological profile of ovarian tumours in Western India. J Med Sci Clin Res. 2016;4:15040-7.
- [Google Scholar]
- A histological study of ovarian tumours in different age groups. Int J Med Sci Public Health. 2014;3:338-41.
- [Google Scholar]
- Histopathological profile of ovarian tumours: A twelve year institutional experience. Muller J Med Sci Res. 2015;6:107-11.
- [Google Scholar]
- Histologic pattern, bilaterality and clinical evaluation of 957 ovarian neoplasms: A 10-year study in a tertiary hospital of Eastern India. J Can Res Ther. 2011;7:433-7.
- [Google Scholar]
- Clinicopathological study of surface epithelial tumours of the ovary: An institutional study. J Clin Diagn Res. 2016;10:EC01-4.
- [Google Scholar]
- Clinicopathological study of ovarian tumours: A 2-year study. Int J Sci Stud. 2017;5:300-5.
- [Google Scholar]
- Histomorphological study of 134 primary ovarian tumors. Adv Lab Med Int. 2011;1:69-82.
- [Google Scholar]
- Immature teratoma of the ovary: A clinicopathological study of 28 cases. Indian J Pathol Microbiol. 2011;54:730-5.
- [Google Scholar]





