Translate this page into:
Effect of Urogenital Cleaning with Paper Soap on Bacterial Contamination Rate While Collecting Midstream Urine Specimens
Address for correspondence: Mr. Narayan Gyawali, E-mail: gyawali123@gmail.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
How to cite this article: Shrestha R, Gyawali N, Gurung R, Amatya R, Bhattacharya SK. Effect of urogenital cleaning with paper soap on bacterial contamination rate while collecting midstream urine specimens. J Lab Physicians 2013;5:17-20.
Abstract
Context:
Urinary tract infection (UTI) is one of the major health problems. Urine culture is considered as a gold standard method for the diagnosis of UTI. But, improper sample collection can lead to contamination with normal urogenital flora. Use of any portable disinfectant that can reduce contamination rate would be the significant help in urine culture interpretation.
Aims:
To observe the effect of urogenital cleaning with paper soap on bacterial contamination rate while collecting specimens.
Materials and Methods:
A cross-sectional comparative study was done in 600 patients aged 15-45 years, equally divided into three groups. The first group was given sterile container and instructed to collect midstream clean catch urine (MSU) after urogenital cleaning with provided piece of paper soap. The second group was given sterile container and strictly instructed to collect the MSU sample after urogenital cleansing by tap water only. The third group was given the sterile container and asked for midstream urine. Collected specimens were inoculated in CLED media, incubated aerobically for overnight at 37°C. Reporting of culture was done according to the guideline of American Society of Microbiology.
Results:
The contamination rate in the three groups were 6.0%, 13.0%, and 27.5%, respectively (P value < 0.05), which was statistically significant.
Conclusions:
Contamination rate was significantly lower in group who provided urine specimen after urogenital cleaning with paper soap. Thus, cleaning the urogenital area may reduce the need of the repeat sample to rule out actual contamination and prevent from the unnecessary antibiotic treatment.
Keywords
Contamination
paper soap
urine culture
INTRODUCTION
Urinary tract infection (UTI) is one of the most common infections. Approximately 10% of human population will suffer from UTI at any time during their life.[1] Urine culture result is considered as the gold standard for diagnosis of UTI.[2] In most instances, growth of ≥ 105 colony forming unit (CFU) per milliliter from a properly collected midstream clean-catch urine (MSU) sample indicates infection.[3] The accurate diagnosis with antibiogram of the isolate is necessary to ensure appropriate therapy. However, improper urine specimen collection can lead to contamination with normal urethral and perineal flora, leading to dilemma in reporting and interpreting the culture growth. Though, MSU collection technique is considered as satisfactory and is most common, [4] many studies have shown varying estimates of the contamination rate (7-31%).[5-8] For every sample contaminant, a repeat sample is warranted to rule out actual contamination. Thus, reports of contaminant delays the confirmation of diagnosis; add to the workload of laboratory may often be a reason for unnecessary antibiotic treatment and the related hazards. Moreover, it becomes economical burden to the patient. Any step that would reduce the contamination rate during midstream urine sampling would be of significant contribution in urine culture interpretation.
Paper soap is a thin soap sheet. It is an anionic surfactant that is used in conjunction with water for washing and cleaning. It is portable, cheap and easy to use.[9] The proposed study aims to assess the effects of perineal/genital (urogenital) cleaning with paper soap on bacterial contamination rates in urine culture, and its affability and acceptance among the patient.
MATERIALS AND METHODS
This cross-sectional comparative study was conducted from March 2010 to July 2010 in 600 patients (participants) aged 15-45 years (adults) who visited Clinical Laboratory Services (CLS) of B. P. Koirala Institute of Health Sciences (BPKIHS), Dharan, Nepal for their urine culture. Random sampling technique was used for the selection of participants. Patients under antimicrobial therapy in the previous seven days were not included in the study. Purpose of the study was clearly described and consent was taken from all participants. Participants consisted of three equal groups. The first group (n = 200) was given sterile container and instructed to collect a MSU urine sample with urogenital cleaning with provided piece of paper soap. Similarly, the second group (n = 200) was given sterile container and instructed to collect a MSU with urogenital cleansing by tap water only. Paper soap was not given to the second group. Likewise, the third group (n = 200) was given the sterile container and asked to collect midstream urine. This later group was not advised to provide clean catch urine specimen. To minimize the errors that are possible with poor understanding of instruction due to verbal communication between researcher and subjects, instruction charts in local (Nepali) language were pasted on wall of sample collection counter of Clinical Laboratory Services and toilets as well. The first group was also asked to rate use of paper soap as convenient, satisfactory, or unsatisfactory.
Thus, collected samples from each group were labeled with patient identification number and transported to microbiology laboratory. The processing of collected urine samples for culture was done by a standard calibrated loop method using cystine lactose electrolyte deficient (CLED) agar media and incubated aerobically at 37°C for 18 - 24 h.
Reporting of culture was done according to the guideline of American Society of Microbiology.[10] If no growth was observed on cultured media after 18 - 24 h it was reported as “no growth < 103 CFU/mL.” If only urogenital or skin microbiota were observed, it was reported as “normal urogenital microbiota grown” and considered as contamination. For low levels (<104 CFU/mL) of organisms commonly found on the skin and external and internal genitalia, mixed growth (three or more than three types of colonies) were reported as contaminants and further suggested for appropriate collection. Only single type or two types of colonies with the count >105 CFU/mL and <105 CFU/mL supported by UTI symptoms were reported as significant growth and followed by the presumptive or definitive identification. However, isolated organism's profile and their susceptibility testing were not recorded for this study purpose.[10]
Statistical analysis used
The collected data were entered in the Excel data entry software and analysis was done using SPSS version 16.0 software. Frequency data comprising age, sex, and culture result was obtained and association of contaminant result in three different sample groups and gender were assessed by using the Chi-square test.
RESULTS
Out of 600 study subjects of three groups of equal number (n = 200), distribution of gender was as follow: first group; 46 males and 154 females, second group; 43 males and 157 females and the third group; 43 males and 157 females. The mean ages of the subjects in these groups were 28.72, 28.56 and 28.99 years, respectively. Number of pure growth, contaminants and no-growth <103 CFU/mL in each group are shown in Table 1.
There was no significant change in contamination rate between genders in either of the groups though the contamination rate is higher in female (P > 0.05).
| Group | Gender | Growth n (%) | Contaminants n (%) | No growth<103 CFU/mL n (%) | Total n (%) |
|---|---|---|---|---|---|
| I | Male | 9 (19.6) | 2 (4.3) | 35 (76.1) | 46 (100.0) |
| Female | 34 (22.1) | 10 (6.5) | 110 (71.4) | 154 (100.0) | |
| II | Male | 7 (15.9) | 4 (9.1) | 32 (75.0) | 43 (100.0) |
| Female | 21 (13.5) | 22 (14.1) | 114 (72.4) | 157 (100.0) | |
| III | Male | 10 (23.3) | 11 (25.6) | 22 (51.20 | 43 (100.0) |
| Female | 36 (22.9) | 44 (28.0) | 77 (49.0) | 157 (100.0) |
Contamination rates in each of the three groups of the subjects were 6.0%, 13.0%, and 27.5% respectively. There was statistical association (P < 0.05) in the contamination rate of these three groups [Figure 1].
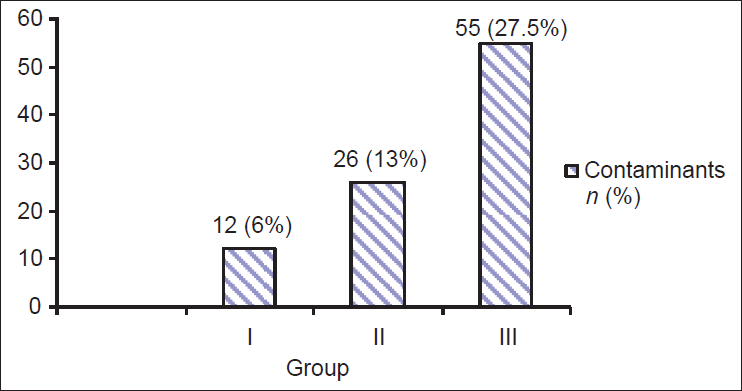
- Comparison of contaminants in three different subject groups (N = 200 in each group) P value=0.002a
Among 200 subjects of the Group I who had used paper soap for cleaning urogenital area, 186 (93%) rated it as convenient and very easy to use, 11 (5.5%) subjects responded as satisfactory, and 3 (1.5%) subjects responded it as unsatisfactory.
DISCUSSION
UTI is one of the most common infections. It occurs when pathogenic microorganisms invade urinary tract, namely urethra, bladder, kidney, or prostate. In most instances, growth of >105 CFU/mL from a properly collected MSU sample indicates infection. Unfortunately, voided urine is invariably contaminated with urethral flora, and in female patients, perineal and vaginal flora, which can confound the results of laboratory testing.[3] Different authors have used different definitions of urine culture contamination.[5,6,8,11] We had defined contamination for the growth of urogenital or skin microbiota, <104 CFU/mL of organisms if not justified by any history and mixed growth of >3 types of organisms.
Varying estimates of the contamination rate in women and men have been shown in different studies.[5,8,11,12] Some of the reported differences can be attributed to differences in the definition of contamination, transportation techniques, and the population under study. Valenstein et al., had found contamination rate twice higher in female, 20.6%, as compared to male, 9.5%.[7] Our study showed higher proportion of contamination in female, 12.66%, as compare to male, 2.83 %. Difference of anatomy of urinary organ in male and female may have played role for higher contamination rate in female.
Studies of Saez-Llorens et al., and Lohr et al., had compared methods of obtaining midstream urine sample in children, and none of these studies found cleaning to reduce the contamination rate.[5,13] However, in our study, we observed that Group I, who were given sterile container and instructed to collect a MSU sample by urogenital cleaning with a sheet of paper soap had lower contamination rate as compared to Group II, who were given sterile container and instructed to collect a MSU sample by urogenital cleaning with tap water and Group III, who were given sterile container and asked for only mid stream urine. Contamination rates found in Group I, Group II, and Group III were 6%, 13%, and 27.5%, respectively. A statistically significant association (P < 0.002) was found among the three groups regarding the contamination rates. These data strongly suggest that the method of urine collection especially by using cleaning materials like paper soap reduce the contamination rate. The result of this study is comparable to the finding of Vailllancourt et al., who had revealed 7.8% of the reduction rate in the group cleaning the urogenital area before collection.[14] The contamination rate of Group III (27.5%) subjects in our study is higher than that of Vaillancourt et al., (23.9%).[14,15]
In our study, 93% of participants from Group I who had used paper soap for cleaning urogenital area rated it as a convenient material and 5.5% of them rated as satisfactory. However, only 1.5% of participants rated as unsatisfactory. Reasons behind rating the paper soap as a convenient material may be its easy use, portability, and quick dissolution in water. Those minorities who rated it as unsatisfactory were perceived it to be an unnecessary step in the collection process.
To our knowledge, no similar study has been carried out on the use of paper soap to reduce contamination of urine sample. Paper soap for urogenital cleaning during collection of urine for culture can be an economical and convenient method. But further studies need to be done for proper evaluation of its feasibility, convenience, and acceptance. However, the study was limited in the area of subject design as those aged 15-45 years visiting OPD were only included in our study. Although verbal instructions were given to the participants and written instructions were pasted on the wall of specimen collection counter and toilets, it was difficult to verify whether the subjects had accurately preformed cleaning as well as midstream urine collection.
Urine culture contamination is a common problem in routine laboratory. Repeated investigations increase the total cost of the test. Very often urine culture contamination leads to unnecessary treatment with poor consequences, like loss of patient's faith toward the treating hospital and laboratory as well as the risk of emergence of resistant pathogens due to antibiotic exposure. Due to its convenient use and potential of reducing the significant level of contamination rates, cleaning the urogital area with paper soap can be recommended as a standard method in MSU collection. However, longitudinal study with the large number of subjects focusing on the contamination rate is necessary to see the effectiveness of the paper soap as a cleaning material to reduce the contamination rates in urine sampling.
Source of Support:
Nil.
Conflict of Interest:
There is no conflict within authors for submitting and publishing of this research article.
REFERENCES
- Urinary tract infections: Disease panorama and challenges. J Infect Dis. 2001;183:S1-4.
- [CrossRef] [PubMed] [Google Scholar]
- Infections of the urinary tract and male genitalia. In: Brillman JC, Quenzer RW, eds. In: Infectious Disease in Emergency Medicine (2nd). Philadelphia: Lippincott-Raven; 1998. p. :601-29.
- [Google Scholar]
- Urinary Tract Infection and Pyelonephritis. In: Harrison's principle of internal medicine (16th). USA: McGraw-Hill; 2005. p. :1715-21.
- [Google Scholar]
- A novel midstream urine-collection device reduces contamination rates in urine cultures amongst women. BJU Int. 2005;96:360-4.
- [CrossRef] [PubMed] [Google Scholar]
- Bacterial contamination rates for non-clean-catch and clean-catch midstream urine collections in uncircumcised boys. J Pediatr. 1989;114:93-5.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of chlorhexadine cleansing in reducing contamination of bagged urine specimens. Can Med Assoc J. 1985;133:1211-3.
- [Google Scholar]
- Urine culture contamination, A Collage Of American Pathologist Q-Probes study of Contaminated Urine Culture in 906 institution. Arch Pathol Lab Med. 1998;122:123-9.
- [Google Scholar]
- Evaluation of urine sampling technique; bacterial contamination of samples from women students. Br J Gen Pract. 1992;42:241-3.
- [Google Scholar]
- Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008;74:3739-44.
- [CrossRef] [PubMed] [Google Scholar]
- Urine culture (Aerobic Bacteriology) In: In: Clinical Microbiology Procedures Handbook Vol 3. (2nd). USA: ASM; 2007. p. :1-4.
- [Google Scholar]
- Bacterial contamination rates for non-clean-catch and clean-catch midstream urine collections in boys. J Pediatr. 1986;109:659-60.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of delay on culture of urine. J Clin Microbiol. 1976;4:102-3.
- [CrossRef] [PubMed] [Google Scholar]
- Bacterial contamination rates in voided urine collections in girls. J Pediatr. 1989;114:91-3.
- [CrossRef] [PubMed] [Google Scholar]
- To clean or not to clean: Effect on contamination rates in midstream urine collections in toilet-trained children. Pediatrics. 2007;119:e1288-93.
- [CrossRef] [PubMed] [Google Scholar]
- Outpatient urine culture: Does collection technique matter? Arch Intern Med. 2000;160:2537-40.
- [CrossRef] [PubMed] [Google Scholar]





