Translate this page into:
Emerging Bacterial Pathogens in the COVID-19 Era: Chryseobacterium gleum—A Case in Point
Address for correspondence: Manisha Biswal, MD, Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh, 160012, India (e-mail: manisha.biswal@gmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction
In the ongoing severe acute respiratory syndrome coronavirus 2 pandemic, a long hospital stay and empirical broad-spectrum antibiotics make the patients prone to acquire nosocomial infections especially with unconventional organisms, and Chryseobacterium gleum is one such rare nosocomial pathogen.
Methods
The given study is a case-series-based study conducted from September 2020 to April 2021 in which clinically suspected pneumonia patients who recovered from coronavirus disease 2019 (COVID-19) were included.
Results
Seventeen C. gleum isolates were obtained in pure culture from the tracheal aspirates of nine COVID-19 patients (including repeat samples to rule out colonization) within a period of eight months (September 2020–April 2021). Our records showed that there has been an increase in the number of isolates of C. gleum obtained in respiratory samples in 2020. We also did a review of literature of all the cases of C. gleum pneumonia reported till now.
Conclusion
To the best of our knowledge, this is the first study reporting the isolation of this rare pathogen from COVID-19 patients with clinical significance in a large cohort of patients. Therefore, it becomes important to consider this pathogen as a significant cause of respiratory infections, especially in patients recovered post COVID-19.
Keywords
emerging
bacterial
COVID-19
Chryseobacterium gleum
pneumonia
Introduction
Chryseobacterium gleum is a nonfermentative, gram-negative bacillus belonging to the family Flavobacterium. It comes under the Centers for Disease Control and Prevention (CDC) group IIb which also comprises Chryseobacterium indologenes and other species within the genus Chryseobacterium.[1] The species in these genera cause healthcare and device-associated infections primarily due to their ability to survive in aqueous environments and by forming biofilms. Majority of the Chryseobacterium species cause infections of the urinary tract and the lower respiratory tract, largely in hospitalized patients with underlying comorbid conditions.[2-5] In the recent times, due to the availability of different diagnostic methods like DNA sequencing and matrix-assisted laser desorption ionization time-of-flight spectrometry (MALDI-TOF MS), it has become easier to differentiate the various species within this genus.
C. gleum was first identified as a medically relevant pathogen in the SENTRY Antimicrobial Surveillance Program.[6] However, in this study, it was the least frequently isolated species within the genus Chryseobacterium, with only two strains identified over the 5-year study period in 16 countries.[6] Though remarkably rare, a growing number of case reports from Europe and Southeast Asia have demonstrated its isolation in respiratory cultures in the recent years.[7]C. gleum has been reported to cause a variety of infections ranging from respiratory tract infections, urinary tract infections, pyonephrosis, septicemia, meningitis, wound infections, to peritonitis.[3,5,8-11] The major worrisome issue with this pathogen is its intrinsic resistance against a broad variety of antibiotics including carbapenems, aminoglycosides, and colistin.[4] The problem of multidrug resistant pathogens has led to an increase in the use of these very drugs, thereby selecting out Chryseobacterium species in patients who are on prolonged treatment with carbapenems and colistin.
In the ongoing severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) pandemic, coronavirus disease 2019 (COVID-19)-induced pneumonia has been commonly seen especially in the high-risk groups, with requirement for prolonged hospitalization. A long hospital stay and empirical broad-spectrum antibiotics make the patients prone to acquire nosocomial infections especially with unconventional organisms and C. gleum is one such rare nosocomial pathogen.[8-11] This study highlights the role of C. gleum in patients who recovered from COVID-19-induced pneumonia and later presented with bacterial pneumonia. To the best of our knowledge, this is the first case series-based study showing ventilator-associated pneumonia (VAP) due to C. gleum in patients recovered post-COVID-19 pneumonia.
Methodology
It is a descriptive retrospective study conducted from September 2020 to April 2021 in which clinically suspected pneumonia patients who recovered from COVID-19 pneumonia were included.
Specimen Processing and Antimicrobial Susceptibility Testing
During the study period, a total of 40 endotracheal aspirate samples from the clinically suspected pneumonia patients who recovered post-COVID-19 pneumonia were received in the microbiology laboratory and processed for aerobic bacterial culture. Samples were inoculated on 5% sheep blood agar and MacConkey agar followed by an overnight incubation at 37°C. Microbiological reporting of the endotracheal samples was done by the semiquantitative method taking greater than 105 colony/mL as significant. To rule out colonization, repeat samples were taken from the same patient. Identification was by MALDI-TOF MS (Vitek MS, Biomerieux Inc., Durham, North Carolina, United States). Antimicrobial susceptibility testing of the isolates was performed on Muller Hinton agar by Kirby Bauer disc diffusion method according to the CLSI 2020 guidelines.[12] Since no standard guidelines for reporting antimicrobial susceptibility testing (AST) for Chryseobacterium spp. were available, we performed AST using antimicrobial agents recommended by the SENTRY antimicrobial surveillance program.[6]
Clinical Correlation
The cases were followed up to establish clinical correlation. The details of changes in treatment and outcome were noted. Analysis was done using the Microsoft Excel version 16 (Microsoft Corp., Richmond, California, United States). An analysis of frequency of isolation of C. gleum in respiratory specimens over the last 5 years, that is, from 2016 to 2020 (prior to the current study period), was also performed.
Environmental Surveillance
To trace the source of infection, environmental surveillance was conducted by the hospital infection control team by sampling the patient areas and their adjoining environment (i.e., ventilator ports, beddings, sterile water from the humidifier, oxygen masks, laryngoscope blades, and ventilator tubings). A total of 100 samples were taken for environmental surveillance. These were further inoculated on 5% sheep blood agar and MacConkey agar. Identification was by MALDI-TOF MS (Vitek MS, Biomerieux Inc., Durham, North Carolina, United States).
Results
Seventeen C. gleum isolates were obtained in pure culture from the tracheal aspirates of nine COVID-19 patients (including repeat samples to rule out colonization) within a period of eight months (September 2020–April 2021). Eight patients were previously positive for COVID-19 and shifted to non-COVID-19 zone of the hospital after getting negative reverse-transcription polymerase chain reaction report for COVID-19 as per the hospital protocol. One patient was detected positive for COVID-19 at the same time when C. gleum was isolated from the endotracheal culture. All the patients were initially admitted to the dedicated COVID-19 area of the hospital and had requirement for ventilatory support due to COVID-19-induced pneumonia. The previous cultures of the endotracheal samples of the eight patients sent during their stay in the COVID-19 hospital were bacteriologically sterile. After being shifted to the non-COVID-19 wards, these patients deteriorated clinically (decreased SpO2, increased oxygen requirement, new-onset fever spikes) as well as radiologically (development of new infiltrates on chest X-ray), due to VAP. C. gleum was isolated in pure culture from the endotracheal aspirates from all the nine patients. The median time of isolation of C. gleum was 15.8 days post-admission and 15.2 days post-mechanical ventilation. Out of the nine patients, four (44.4%) succumbed to the illness. A detailed description of the clinical courses and laboratory parameters is provided in ►Table 1. An environmental source for this organism could not be identified as C. gleum was not isolated from any of the environmental sample cultured. Of this cohort, seven patients had received colistin and five vancomycin and five meropenem empirically.
| Age in years/Gender | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 |
|---|---|---|---|---|---|---|---|---|---|
| 70/ female | 74/ male | 50/ female | 28/ female | 49/ female | 59/ female | 36/ male | 59/ female | 37/ male | |
| Symptoms on presentation | Fever and severe respiratory distress for last 2 days | Fever and shortness of breath for last 5 days | Fever along with chills, myalgia for last 5 days, shortness of breath for 1 day | Fever, cough for 14 days and shortness of breath for 4 days | Dry cough for 2 weeks, fever for 2 days and shortness of breath for 1 day | Abdominal pain and vomiting for last 2 days, altered sensorium, respiratory distress and decreased urine output for last 1 day | Fever and cough along with shortness of breath for 4 days | Fever and cough for last 10 days along with shortness of breath for last 3 days | Chest pain, cough associated with few streaks of blood for 3 days, fever, shortness of breath for 2 days |
| Time period between mechanical ventilation and culture positive for Chryseobacterium gleum | 14 days | 16 days | 13 days | 10 days | 10 days | 20 days | 24 days | 24 days | 6 days |
| Clinical picture at the time of isolation of bacteria | New onset fever, purulent tracheal secretions, increase in daily FiO2 requirement, leucocytosis | New onset fever, purulent tracheal secretions, leucocytosis, bronchial breath sounds, worsening gas exchange | New onset fever, purulent tracheal secretions, leucocytosis | New onset fever, purulent tracheal secretions, bronchial breath sounds | New onset fever, purulent tracheal secretions, leucocytosis, worsening gas exchange | New onset fever, purulent tracheal secretions, leucocytosis, bronchial breath sounds | New onset fever, purulent tracheal secretions, leucocytosis, bronchial breath sounds, worsening gas exchange | New onset fever, purulent tracheal secretions, leucocytosis, worsening gas exchange | New onset fever, purulent tracheal secretions, leucocytosis, bronchial breath sounds |
| Final diagnosis | Post-COVID-19, ARDS, VAP | Post-COVID-19, ARDS, VAP | Post COVID-19, ARDS, VAP | Post-COVID-19, ARDS, VAP | Post-COVID-19, ARDS, VAP | Acute necrotizing pancreatitis, VAP | Post-COVID-19, ARDS, VAP | Post-COVID-19, ARDS, VAP | Post-COVID-19 ARDS, VAP |
| Comorbidities | Type 2 DM, HTN | Type 2 DM, HTN | Type 2 DM, HTN | Myasthenia gravis, post-thymectomy, on immunosuppressants | None | Type 2 DM | None | Type 2 DM, HTN | Type 2 DM |
| Empirical antibiotics | Colistin, meropenem | Colistin, vancomycin | Colistin, vancomycin | Colistin, meropenem | Meropenem, vancomycin | Colistin, vancomycin | Meropenem | Colistin, meropenem | Colistin, vancomycin |
| Radiology | New onset bilateral chest infiltrates-lower lobe of lung | Left lower lobe consolidation, opacity | Bilateral diffuse alveolar opacities | Bilateral dense patchy consolidations, bilateral minimal pleural effusion, bilateral ground glass opacities | Bilateral diffuse peribronchovascular consolidation along with thick-walled cavity on right lower lobe region | Bilateral chest infiltrates in the lower lobe of lung | New onset bilateral chest infiltrates, prominent reticular markings | Left lower lobe consolidation, left lower lobe infiltrates | Bilateral chest infiltrates in the lower lobe of lung |
| Sensitivity of Chryseobacterium gleum | Sensitive to: ceftazidime, chloramphenicol, cotrimoxazole, meropenem, minocycline, levofloxacin, piperacillin-tazobactam, ciprofloxacin, tetracycline Resistant to: Amikacin, meropenem |
Sensitive to: ceftazidime, chloramphenicol, cotrimoxazole, minocycline, levofloxacin, piperacillin-tazobactam, ciprofloxacin, tetracycline Resistant to: meropenem, amikacin |
Sensitive to: ceftazidime, chloramphenicol, cotrimoxazole, minocycline, levofloxacin, piperacillin-tazobactam, ciprofloxacin, tetracycline Resistant: Amikacin, meropenem |
Sensitive to: ceftazidime, cotrimoxazole, minocycline, levofloxacin, piperacillin–tazobactam, ciprofloxacin, tetracycline Resistant to: chloramphenicol, amikacin, meropenem |
Sensitive to: cotrimoxazole, minocycline, piperacillin–tazobactam, ciprofloxacin, tetracycline Resistant to: amikacin, meropenem, chloramphenicol, levofloxacin, ceftazidime |
Sensitive to: ceftazidime, chloramphenicol, cotrimoxazole, minocycline, levofloxacin, piperacillin–tazobactam, ciprofloxacin, tetracycline Resistant to: Amikacin, meropenem |
Sensitive to: ceftazidime, cotrimoxazole, minocycline, levofloxacin, piperacillin–tazobactam, ciprofloxacin, tetracycline Resistant to: Amikacin, meropenem, chloramphenicol |
Sensitive to: ceftazidime, chloramphenicol, cotrimoxazole, minocycline, levofloxacin, piperacillin–tazobactam, ciprofloxacin, tetracycline Resistant to: Amikacin, meropenem |
Sensitive to: chloramphenicol, cotrimoxazole, minocycline, levofloxacin, piperacillin–tazobactam, ciprofloxacin Resistant to: Amikacin, meropenem, ceftazidime |
| Blood culture | Sterile | Sterile | Sterile | Sterile | Sterile | Sterile | Sterile | Acinetobacter baumannii | Sterile |
| TLC (4,000–11,000/L) | 19,800 | 17,700 | 19,500 | 7,400 | 20,000 | 20,000 | 13,000 | 15,000 | 15,000 |
| Neutrophils (41–72%) | 85.9 | 89 | 78.2 | 90.5 | 90 | 84 | 87 | 90 | 88 |
| Procalcitonin (0.01–0.50 ng/mL) | 2.79 | 2.71 | 0.864 | 5.3 | 2.2 | 8.1 | 2.2 | 1.81 | 29.8 |
| CRP (0–5 mg/L) | 73.03 | 184.7 | 270.04 | 409 | 333.3 | 170.22 | 150.2 | 24.2 | 155.55 |
| PT/INR (80–100% | 100/0.9 | 90/1.29 | 70/1.4 | 96/1.04 | 70/0.8 | 96/1.3 | 78/1.28 | 76/1.3 | 98/1.4 |
| Treatment given | Cotrimoxazole, ceftazidime | Levofloxacin | Ceftazidime, piperacillin–tazobactam | Ceftazidime, piperacillin–tazobactam | Piperacillin–tazobactam | Minocycline, piperacillin–tazobactam | Levofloxacin | Ceftazidime | Levofloxacin, piperacillin tazobactam |
| Outcome | Discharged under stable conditions | Died (post-COVID-19, septic shock) | Discharged under stable conditions | Discharged under stable conditions | Died (septic shock, VAP, post-COVID-19 ARDS) | Discharged under stable conditions | Died (post-COVID-19 ARDS, VAP, upper GI bleeding) | Died (septic shock, VAP, post-COVID-19 ARDS) | Discharged under stable conditions |
Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; DM, diabetes mellitus; FiO2, fraction of inspired oxygen; GI, gastrointestinal; HTN, hypertension; INR, international normalized ratio; PT, prothrombin time; TLC, total leukocyte count; VAP, ventilator-associated pneumonia.
Our records showed that there has been an increase in the number of isolates of C. gleum obtained in respiratory samples in 2020 (►Fig. 1, after excluding repeat samples from same patients). We also observed that during the study period, out of a total of 10 patients having positive respiratory cultures for C. gleum, nine (90%) had history of COVID-19 infection (►Fig. 2).
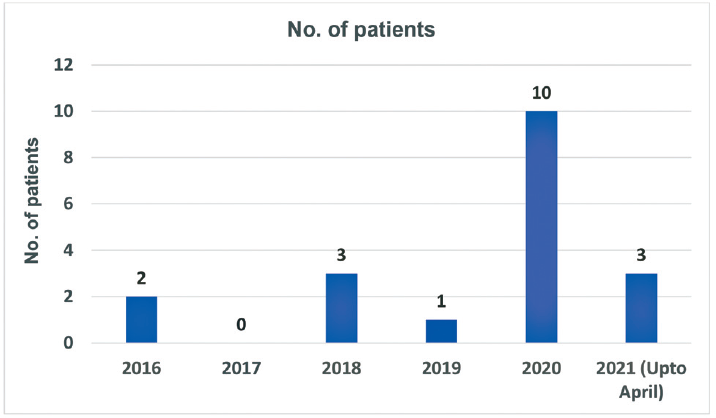
- Comparison of number of patients having Chryseobacterium gleum with previous years.
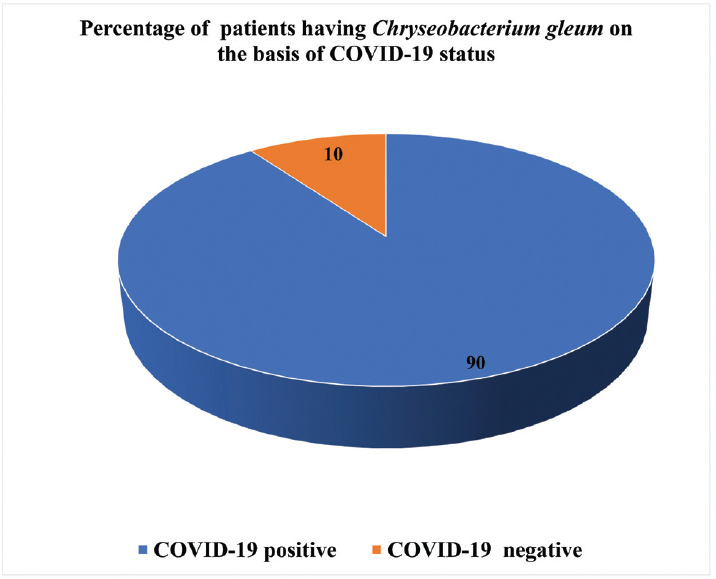
- Percentage of total number of patients having Chryseobacterium gleum pneumonia based on coronavirus disease 2019 status during the study period.
Discussion and Review of Literature
We found C. gleum to be the causative agent for VAP in nine subjects with COVID-19 disease. Till now there were no reports of the isolation of C. gleum in cases of VAP from patients post-COVID-19 infection. To the best of our knowledge, this is the first study reporting the isolation of this rare pathogen from previously positive COVID-19 patients with clinical significance in a large cohort of patients. Therefore, it becomes important to consider this pathogen as a significant cause of respiratory infections especially in patients recovered post-COVID-19.
Chryseobacterium spp. are emerging gram-negative bacilli belonging to the family of nonfermenters. More than 100 species have been reported in the genus Chryseobacterium but only C. indologenes and C. gleum have been the most isolated species from humans. In the past, these bacteria were mainly isolated from patients with polymicrobial infections that made it difficult to determine their clinical significance.[6,13] However, in recent times this organism has been isolated in pure cultures from various patient samples, suggesting an emerging role of this organism in causing disease in humans. MALDI-TOF MS has also assisted in the better identification of the various species within the genus Chryseobacterium.[3,5,7,8] In fact, a study by Lo and Chang found MALDI-TOF to be an excellent and cost-effective alternative to 16s rRNA sequencing for its ability to identify C. gleum.[8]
This organism has been mainly associated with hospital-acquired infections (HAIs).[3,5,6,13] Factors like the inherent chlorine resistance of this genus facilitate its persistence within the hospital environment and thereby increasing its likelihood in causing nosocomial infections.[14] Many of the Chryseobacterium spp. especially C. indologenes are well known for their intrinsic resistance to carbapenems and cephalosporins due to the presence of class A and B β-lactamases.[15,16] A study by Bellais et al reported heterogeneity in metallo β-lactamases in Chryseobacterium species.[16]Chryseobacterium spp. are well known to colonize various medical devices containing fluids such as respirators, humidifiers, and syringes. Many COVID-19 cases require prolonged hospitalization. Along with an increase in need for mechanical ventilation, there is an increased risk of various HAIs due to rare and opportunistic organisms such as C. gleum. During the given period, a total of 18 isolates of C. gleum had been obtained from 10 patients, out of which 17 isolates were from nine COVID-19 positive patients, suggesting a possible association between COVID-19 virus and C. gleum respiratory tract infection. Our systematic review yielded only 18 reports of C. gleum from different clinical specimens like blood, sputum, urine, and pus.[3,5,7-10,17-28] In a previously published study from our institute, 19 Chryseobacterium species were isolated from urine samples in patients with urological complaints, of which 15 belonged to Chryseobacterium indologenes and only four to C. gleum.[22] Similarly, in a recent study from North India, 20 isolates of Chryseobacterium spp. were identified over a span of 3 years (2017–2019),[23] where C. indologenes (18/20) was the commonest species followed by C. gleum (2/20), although clinical correlation could not be established for the C. gleum isolates. As far as the role of C. gleum as a causative agent of respiratory infections is concerned, only ten cases have been reported till now.[3,7-9,19-21,25,27,28] A detailed review of all these cases has been provided in ►Table 2.
| Author | Year | Country | Susceptibility testing interpretive criteria | Susceptibility profile | Treatment | Response |
|---|---|---|---|---|---|---|
| Lambiase et al[25] | 2007 | Italy | NCCLS: not specified | #Resistant: AMK, ATM, CAZ, CTX, FEP, GEN, IPM, MEM, SAM, TZP Susceptible: CIP, LVX, SXT |
NA | NA |
| Virok et al[3] | 2014 | Hungary | EUCAST: Pseudomonas spp. | #Resistant: AMK, DOR, GEN, IPM, MEM, TOB, TZP Susceptible: CAZ, CIP, FEP, LVX |
CIP | Responded |
| Lo and Chang[8] | 2014 | Taiwan | CLSI: other non-Enterobacteriaceae | #Resistant: AMK, AMS, CAZ, CFZ, CRO, CST, FEP, FOX, GEN, IPM, PIP, TZP Susceptible: CIP, MIN, SXT, TGC |
NA | NA |
| Brkic et al[9] | 2015 | Croatia | EUCAST: Gram-negative non-fermentative bacteria | Resistant: CST, DAP, IPM, MEM, VAN Susceptible: CAZ, CIP, FEP, TGC, TZP |
TZP | Responded |
| Abdalhamid et al[19] | 2016 | Saudi Arabia | CLSI: other non-Enterobacteriaceae | Resistant: AMK, CAZ, CIP, CST, FEP, GEN, IPM, MEM, TGC, TZP, VAN Susceptible: LVX, MIN, SXT |
LVX | Responded |
| Rawat et al[20] | 2017 | India | NA | Resistant: NA Sensitive: MIN, SXT, TZP |
TZP + SXT | Responded |
| Jain et al[7] | 2017 | India | CLSI: other non-Enterobacteriaceae | Resistant: AMX, CAZ, CFP, CLI, CRO, CST, CTX, DOX, ERY, FEP, GEN, IPM, MEM, TOB Sensitive: AMK, CIP, DOX, LVX, MIN, SXT, TZP, VAN |
LVX | Responded but later on died due to sudden cardiac arrest |
| Mirza et al[21] | 2018 | Turkey | CLSI: other non-Enterobacteriaceae | Resistant: AMK, GEN, IPM, MEM Sensitive: CAZ, CIP, FEP, LVX, SXT, TZP |
NA | NA |
| Tsouvalas et al[28] | 2020 | USA | CLSI: non-fermentative Gram-negative bacilli | Resistant: AMK, ATM, CAZ, CRO, FEP, GEN, IPM, MEM, TOB Sensitive: SXT |
SXT | Responded |
| Amisha et al[27] | 2021 | USA | NA | Resistant: TGC, GEN, TOB Sensitive: AMK, FEP, CAZ, CRO, LVX, MEM, TZP, SXT |
SXT, LVX | Died |
Abbreviations: AMK, amikacin; AMX, amoxicillin; ATM, Aztreonam; CAZ, ceftazidime; CFP, cefoperazone; CFZ, cefazolin; CIP, ciprofloxacin; CLI, clindamycin; CLSI, Clinical and Laboratory Standards Institute; CRO, ceftriaxone; CST, colistin; CTX, cefotaxime; DAP, daptomycin; DOR, doripenem; DOX, doxycycline; ERY, erythromycin; EUCAST, European Committee on Antimicrobial Susceptibility Testing; FEP, cefepime; FOX, cefoxitin; GEN, gentamicin; IPM, imipenem; LVX, levofloxacin; MEM, meropenem; MIN, minocycline; NA, not applicable; NA, not available; NCCLS, National Committee for Clinical Laboratory Standards; PIP, piperacillin; SAM, ampicillin–sulbactam; SXT, trimethoprim–sulfamethoxazole; TGC, tigecycline; TOB, tobramycin; TZP, piperacillin–tazobactam; VAN, vancomycin.
#Multiple strains reported.
In the present case series, C. gleum was isolated in pure culture from the tracheal aspirates of nine patients and the isolation correlated clinically as well as radiologically. Comorbid conditions like diabetes mellitus, chronic kidney disease, cardiovascular disease, chronic obstructive pulmonary disease, and malignancies were associated risk factors for acquiring infections with this pathogen.[15] Similar findings were seen in the present cases where seven patients (77.8%) had underlying comorbidities. Other risk factors associated with the isolation of C. gleum as reported in literature[14,15,26] include exposure to broad-spectrum antibiotics like vancomycin and colistin, prolonged hospitalization, invasive interventions, and medical intensive care unit (ICU) stay (greater than 21 days).[14,27,29]
The organisms in the Chryseobacterium genus are well known to form biofilms on various medical devices[15,28] and thus causing device-related HAIs. All the patients in the given study had a history of a prolonged hospital stay including ICU admission, mechanical devices, presence of a central line port, endotracheal tube, and invasive ventilation. In our patients, seven patients had more than 14 days duration of hospital stay before the isolation of C. gleum and two of them had more than 21 days of hospitalization. We found C. gleum as a causative agent of VAP in nine subjects with severe COVID-19 disease. We also found a high mortality due to C. gleum. C. gleum is intrinsically resistant to most antibiotics (carbapenems, colistin) that are used empirically to treat HAIs. Most secondary HAIs in our institute are due to Acinetobacter baumannii that is sensitive to carbapenems and colistin. In all our patients, we empirically started colistin on clinical worsening based on our previous antibiogram. We treated C. gleum only after receiving the final culture and sensitivity report. This may have resulted in a delay in initiating the appropriate antibiotics and could explain a high mortality in our series. We have now changed our antibiotic prescription and usually add cotrimoxazole, fluoroquinolones, or ceftazidime in addition to carbapenems or colistin. Centers involved in caring for COVID-19 subjects should investigate all HAIs for finding unusual microorganism and modify their antibiotic policy accordingly.
Recent studies have reported increasing incidence of secondary bacterial infections (SBIs) in COVID-19 patients.[30-32] The most commonly isolated bacteria are Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas species. In none of these studies Chryseobacterium spp has been isolated as a cause of SBIs. Primarily, the decreased airway defense function after a SARS-CoV2 infection is one very important factor contributing to the acquisition of SBIs.[31] Several divergent inflammatory pathways in COVID-19 act together to produce an inflammatory environment leading to development of different superinfections.[33] SARS-CoV2 suppress type 1-interferon production, which further compromises the alveolar macrophage recruitment and function. Downregulation and differential regulation of immune genes are mechanisms that may create a positive environment for the establishment of SBIs,[34,35] favoring bacterial attachment to host structural cells and proinflammatory environment leading to suppression of antibacterial host defenses. Therefore, it can be hypothesized that SARS-CoV2-induced immunomodulatory effects in the respiratory epithelium can predispose the patients to this rare pathogen along with other risk factors like a prolonged hospital stay, underlying comorbid conditions, and mechanical ventilation. Pointed studies are needed to confirm the exact pathogenesis of rare organisms like C. gleum in causation of pneumonia in a scenario of post-COVID-19 pneumonia.
One important issue in such cases is how to differentiate a true pathogen from a colonizer especially when there is a history of prolonged hospitalization and mechanical ventilation.[36] Isolation of the same organism in pure culture with high bacterial count and in repeat samples along with the clinical picture supports the notion that an isolated microbe is a pathogen and cannot be ignored as a mere contaminant, but no specific guidelines for repeat cultures exist in the literature.[7] In our series, the respiratory secretions grew C. gleum on repeat cultures. Also, all subjects had clinical worsening suggesting C. gleum to be responsible for the clinical worsening. Moreover, 89% (8/9) of the patients had prior respiratory cultures where C. gleum was not isolated, thereby ruling out a carrier state. To the best of our knowledge, there is no evidence of a respiratory carrier state of C. gleum documented in the literature.
No standard guidelines are available from either the CLSI (Clinical and Laboratory Standards Institute) or the EUCAST (European Committee on Antimicrobial Susceptibility Testing) for the antimicrobial susceptibility testing of members within the genus Chryseobacterium. Some studies have used Staphylococcus breakpoints for minimal inhibitory concentration interpretation,[4] while others have chosen nonfermenting gram-negative bacilli cutoffs to interpret results.[17,19] The SENTRY study (1997–2001) estimated the epidemiology and antimicrobial susceptibility pattern of Chryseobacterium infections worldwide, where the most active antimicrobials were the newer quinolones (garenoxacin, gatifloxacin, and levofloxacin), followed by rifampin, trimethoprim–sulfamethoxazole, ciprofloxacin, and piperacillin–tazobactam.[6] In the given case series, the isolates were sensitive to most of the antibiotics. Maximum resistance was seen against amikacin and meropenem followed by chloramphenicol. Previous studies have tested the effects of vancomycin and clindamycin on these bacteria as an identification marker and not for treatment purposes.[5,6] As in the previous case reports, the organism has consistently shown susceptibility to trimethoprim–sulfamethoxazole, fluoroquinolones such as levofloxacin and ciprofloxacin or piperacillin–tazobactam (or a combination of these agents), these agents are typically used for therapy.[5,7,8,19,21,27,37] However, in our patients levofloxacin was used in three patients and most commonly used antibiotic was piperacillin–tazobactam and ceftazidime.
One interesting observation in the present case series was that the outcome when a single antibiotic was given was poor when compared with those who received a combination of agents. Previous studies in non-COVID-19 patients have shown that treatment by a single antimicrobial agent (piperacillin–tazobactam, quinolones or tetracycline) is curative. Whereas as observed in our cases of COVID-19 patients there can be a possibility that in the already compromised lungs due to the virus-mediated direct damage to the lung epithelium, an intense host immune response in the form of an aberrant cytokine storm required an intense regimen to combat the bacterial burden.[33,34]
The isolates showed different antibiotic sensitivity profiles and C. gleum were not detected in the environmental samples suggesting thereby that the infections did not originate from a point source. Moreover, in all the cases, similar organism was not isolated from blood cultures indicating a localized respiratory infection without disseminated infection. One case report till now has reported simultaneous isolation of C. gleum from both blood and tracheal aspirate.[7] The previous studies have not reported mortality in cases with C. gleum. However, in our case series, 44.4% patients died and the cause of death in all these patients was septic shock following VAP, so this is the first report to our knowledge documenting the mortality associated with this new emerging nosocomial pathogen. Therefore, it becomes important to consider this pathogen as a significant cause of respiratory infections especially in patients recovered post-COVID-19.
Conclusion
Our report is the first to establish the association of C. gleum with COVID-19 infection as a cause of SBI in this group of patients. This study is also the first to establish the mortality associated with this new emerging pathogen. Critically ill patients in ICUs, with mechanical devices, receiving broad-spectrum antibiotics are at risk of developing healthcare-associated infections due to this pathogen. Since it is inherently resistant to carbapenems and colistin, its rapid and accurate identification in the laboratory, preferably based on MALDI-TOF MS, is essential for guiding therapy. Moreover, standard in vitro susceptibility methods for this rare organism should be established that can be applied in routine microbiological practices. However, more extensive studies highlighting the exact mechanisms of pathogenesis are required.
Limitations
Molecular techniques could not be performed to say the given case series as an outbreak as we could not retrieve some isolates of C. gleum.
Ethical Approval
The study was approved by the Institute Ethics Committee with reference no. NK/6623/Study/057.
Conflict of Interest
None declared.
Funding
None.
References
- Subcommittee On The Taxonomy Of Flavobacterium And Cytophaga-Like Bacteria Of The International Committee On Systematics Of Prokaryotes. Proposed minimal standards for describing new taxa of the family Flavobacteriaceae and emended description of the family. Int J Syst Evol Microbiol. 2002;52(Pt 3):1049-1070.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical impact of Gram-negative nonfermenters on adults with community-onset bacteremia in the emergency department. J Microbiol Immunol Infect. 2015;48(01):92-100.
- [CrossRef] [PubMed] [Google Scholar]
- Chryseobacterium gleum - a novel bacterium species detected in neonatal respiratory tract infections. J Matern Fetal Neonatal Med. 2014;27(18):1926-1929.
- [CrossRef] [PubMed] [Google Scholar]
- Chryseobacterium indologenes non-catheter-related bacteremia in a patient with a solid tumor. J Clin Microbiol. 2005;43(04):2021-2023.
- [CrossRef] [PubMed] [Google Scholar]
- Pyonephrosis due to Chryseobacterium gleum: a first case report. Indian J Med Microbiol. 2015;33(02):311-313.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial susceptibility and epidemiology of a worldwide collection of Chryseobacterium spp: report from the SENTRY Antimicrobial Surveillance Program (1997-2001) J Clin Microbiol. 2004;42(01):445-448.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous isolation of Chryseobacterium gleum from bloodstream and respiratory tract: first case report from India. JMM Case Rep. 2017;4(10):e005122.
- [CrossRef] [PubMed] [Google Scholar]
- Identification, characterization, and biofilm formation of clinical Chryseobacterium gleum isolates. Diagn Microbiol Infect Dis. 2014;79(03):298-302.
- [CrossRef] [PubMed] [Google Scholar]
- Chryseobacterium gleum infection in patient with extreme malnutrition and hepatic lesion-case report. Signa Vitae. 2015;10:50-52.
- [CrossRef] [Google Scholar]
- Flavobacterium gleum, a new species found in human clinical specimens. Int J Syst Bacteriol. 1984;34(01):21-25.
- [CrossRef] [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing. In: CLSI supplement M100 (30th). Wayne: Clinical and Laboratory Standards Institute; 2020.
- [Google Scholar]
- Road map of the phyla Bacteroidetes, Spirochaetes, Tenericutes (Mollicutes), Acidobacteria, Fibrobacteres, Fusobacteria, Dictyoglomi, Gemmatimonadetes, Lentisphaerae, Verrucomicrobia, Chlamydiae, and Planctomycetes. In: Krieg N, Parte A, Ludwig W, Whitman W, Hedlund B, Paster B, eds. Bergey's Manual of Systematic Bacteriology. Vol 4. New York: Springer Science & Business Media; 2011. p. :1-19.
- [CrossRef] [Google Scholar]
- Flavobacterium indologenes bacteremia: clinical and microbiological characteristics. Clin Infect Dis. 1996;23(03):550-555.
- [CrossRef] [PubMed] [Google Scholar]
- Differences in clinical manifestations, antimicrobial susceptibility patterns, and mutations of fluoroquinolone target genes between Chryseobacterium gleum and Chryseobacterium indologenes. Antimicrob Agents Chemother. 2019;63(05):e02256-e18.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic diversity of carbapenem-hydrolyzing metallo-beta-lactamases from Chryseobacterium (Flavobacterium) indologenes. Antimicrob Agents Chemother. 2000;44(11):3028-3034.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary tract infection due to Chryseobacterium gleum, an uncommon pathogen. Indian J Pathol Microbiol. 2016;59(04):551-553.
- [CrossRef] [PubMed] [Google Scholar]
- Discrepancy in MALDI-TOF MS identification of uncommon Gram-negative bacteria from lower respiratory secretions in patients with cystic fibrosis. Infect Drug Resist. 2015;8:83-88.
- [CrossRef] [PubMed] [Google Scholar]
- Chryseobacterium gleum pneumonia in an infant with nephrotic syndrome. IDCases. 2016;5:34-36.
- [CrossRef] [PubMed] [Google Scholar]
- Infection profile in chronic granulomatous disease: a 23-year experience from a tertiary care center in North India. J Clin Immunol. 2017;37(03):319-328.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical Strains of Chryseobacterium and Elizabethkingia spp. Isolated from pediatric patients in a university hospital: performance of MALDI-TOF MS-Based identification, antimicrobial susceptibilities, and baseline patient characteristics. Microb Drug Resist. 2018;24(06):816-821.
- [CrossRef] [PubMed] [Google Scholar]
- Increased recognition of Chryseobacterium species as an emerging cause of nosocomial urinary tract infection following introduction of matrix-assisted laser desorption/ionisation-time of flight for bacterial identification. Indian J Med Microbiol. 2017;35(04):610-616.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical correlation and antimicrobial susceptibility pattern of Chryseobacterium spp.: a three year prospective study. Intractable Rare Dis Res. 2021;10(01):37-41.
- [CrossRef] [PubMed] [Google Scholar]
- Sepsis due to Chryseobacterium gleum in a diabetic patient with chronic obstructive pulmonary disease: a case report and mini review. Jpn J Infect Dis. 2017;70(06):687-688.
- [CrossRef] [PubMed] [Google Scholar]
- Chryseobacterium respiratory tract infections in patients with cystic fibrosis. J Infect. 2007;55(06):518-523.
- [CrossRef] [PubMed] [Google Scholar]
- Chryseobacterium gleum in a man with prostatectomy in Senegal: a case report and review of the literature. J Med Case Reports. 2017;11(01):118.
- [CrossRef] [PubMed] [Google Scholar]
- Chryseobacterium gleum causing healthcare-associated pneumonia in an adult male with diffuse large B cell lymphoma. Cureus. 2021;13(11):e19297.
- [CrossRef] [PubMed] [Google Scholar]
- Chryseobacterium gleum isolation from respiratory culture following community-acquired pneumonia. Am J Case Rep. 2020;21:e921172.
- [CrossRef] [PubMed] [Google Scholar]
- Chryseobacterium meningosepticum: an emerging pathogen among immunocompromised adults. Report of 6 cases and literature review. Medicine (Baltimore). 1997;76(01):30-41.
- [CrossRef] [PubMed] [Google Scholar]
- Profile of co-infections & secondary infections in COVID-19 patients at a dedicated COVID-19 facility of a tertiary care Indian hospital: implication on antimicrobial resistance. Indian J Med Microbiol. 2021;39(02):147-153.
- [CrossRef] [PubMed] [Google Scholar]
- Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: a retrospective analysis. Antimicrob Resist Infect Control. 2020;9(01):153.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dis. 2020;20(01):646.
- [CrossRef] [PubMed] [Google Scholar]
- The Emergence of COVID-19 Associated Mucormycosis: Analysis of Cases From 18 Countries. 2021 May 12 Accessed July 10, 2022 at SSRN: https://ssrn.com/abstract=3844587 or http://dx.doi.org/10.2139/ssrn.3844587
- [Google Scholar]
- SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol Med. 2020;12(07):e12560.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular pathogenesis of secondary bacterial infection associated to viral infections including SARS-CoV-2. J Infect Public Health. 2020;13(10):1397-1404.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic tests for agents of community-acquired pneumonia. Clin Infect Dis. 2011;52(Suppl 4):S296-S304.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and microbiological characteristics of Flavobacterium indologenes infections associated with indwelling devices. J Clin Microbiol. 1996;34(08):1908-1913.
- [CrossRef] [PubMed] [Google Scholar]






