Translate this page into:
Epidermal Growth Factor Receptor Expression in Triple Negative and Nontriple Negative Breast Carcinomas
Address for correspondence: Dr. Arathi A Changavi, E-mail: aarathi_p@in.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
The panel of markers used for molecular classification include estrogen receptors (ER), progesterone receptors (PR), human epidermal growth factor receptor (HER)-2/neu, p53, Bcl-2 and basal markers like cytokeratin 5/6 or epidermal growth factor receptor (EGFR). Among these, EGFR plays an important role and is associated with bad prognosis.
Aims and Objectives:
To study EGFR expression in triple negative breast carcinoma (TNBC) and non-TNBCs (NTNBCs).
Materials and Methods:
Fifty cases of breast carcinomas were classified and graded according to World Health Organization and Nottingham modification of Scarff-Bloom-Richardson (SBR) system, respectively. The age of the patients ranged from 28 to 69 years. Histological features such as necrosis, pushing borders, lymphocytic infiltrate and periductal elastosis were noted. The panel of markers used in our study included ER, PR, HER-2/neu and EGFR. EGFR expression was assessed based on membrane staining. Chi-square test was applied for statistical analysis to compare EGFR expression with hormonal status and prognostic factors. P < 0.05 was considered significant.
Results:
The mean age was 49.8 ± 7.2 years. There were 44 (88%) infiltrating ductal carcinoma, 3 (6%) medullary carcinoma and 3 (6%) mucinous carcinoma. EGFR expression was common in young patients and was predominant in TNBC (89.47%), was also expressed in few cases of NTNBC. There was a positive correlation of EGFR expression (P = 0.03491) with a high grade. Medullary carcinomas were triple negative and strongly expressed EGFR. EGFR expression was inversely associated with ER status and showed strong association with necrosis and lymphocytic infiltrate, but not with pushing border and periductal elastosis.
Conclusion:
EGFR is an important marker to stratify patients with breast cancer according to molecular classification. Its expression correlated positively with young age, higher SBR grade, necrosis, lymphocytic infiltrate and inversely with hormonal receptor expression.
Keywords
Epidermal growth factor receptor
hormonal status
nontriple negative breast carcinoma
triple negative breast carcinoma
INTRODUCTION
Breast cancer is the most frequent malignant tumor in females. A significant improvement was the introduction of new molecular classification that recognized five types of breast carcinoma: Luminal A, luminal B, basal-like, human epidermal growth factor receptor (HER)-2/neu and unclassified. The main goal of this classification was to clarify the molecular markers of prognosis and to show the potential response to adjuvant therapy. The five molecular types of breast cancer were demonstrated by gene profile analysis, and immunohistochemistry (IHC) was shown to be a good and accurate surrogate to define a particular type.[1]
The panel of markers for molecular classification of breast cancers include estrogen receptors (ER), progesterone receptors (PR), HER-2/neu, p53, Bcl-2 and basal markers like cytokeratin 5/6 or epidermal growth factor receptor (EGFR). Among these, EGFR plays an important role and can be a target for specific inhibitors.[2]
The epidermal growth factor receptor is a tyrosine kinase receptor in the HER family which is widely expressed in a number of epithelial tumors and is believed to play a key role in cell proliferation.[3] EGFR activation results in cell proliferation, survival, angiogenesis, invasion, and metastasis. Aberrant expression of EGFR has been linked to etiology of many human epithelial cancers such as breast cancer, head and neck squamous cell carcinoma, nonsmall cell lung, colorectal, pancreatic and brain cancers.[4]
Expression of EGFR is seen in 15–45% of breast cancer.[5] Its expression was found mainly in basal-like carcinoma, but many studies have reported positive cases associated with HER-2/neu positive or luminal B (basoluminal) types.[1] EGFR expression is more common in breast tumors in younger women and is associated with lower hormone receptor (ER, PR) levels, higher proliferation, genomic instability, and HER-2/neu overexpression. It is correlated with higher risk of relapse and bad prognosis in patients receiving adjuvant treatment. Blocking EGFR may improve outcome in selected patients.[5] Medullary carcinomas also express basal markers, but its prognosis is better compared to nontriple negative tumors.
Our present study aims to classify the breast carcinoma based on new molecular classification and to analyze EGFR expression in triple negative and nontriple negative breast carcinomas (NTNBC).
MATERIALS AND METHODS
In a cross-sectional study, 50 cases of invasive breast carcinoma were included in the study. Clinical details regarding age, tumor size and lymph node status were collected from case records. This study was conducted after obtaining approval from the ethical committee and informed consent from the patient. For the histopathological study, the sections were stained with hematoxylin and eosin. The tumors were classified and graded according to World Health Organization and Nottingham modification of Scarff-Bloom-Richardson (SBR) system, respectively. Other histopathological features like presence or absence of necrosis, pushing borders, lymphocytic infiltrate and periductal elastosis were noted.
The panel of markers used was ER, PR, HER-2/neu and EGFR. For IHC, 4 µm sections from representative areas were attached on poly-lysine coated slides and were incubated at 37°C. The slides were dewaxed in xylene, rehydrated in graded alcohol and covered with 10 mm citrate buffer (pH 6). The antigen retrieval was done and was then incubated for 30 min with primary monoclonal antibodies against HER-2, ER, PR and EGFR. This was followed by incubation with secondary antibodies. For each run of staining, a positive control slide was included.
The sections were assessed for ER and PR by Quick score. For the present study, 1% of the cells showing ER positivity were considered as positive. For assessing HER-2-neu, the American society of clinical oncology and the College of American pathologist have provided guidelines (2013) which were followed. For EGFR reporting, only membrane staining was assessed according to DAKO criteria [Table 1].

For statistical analysis, to compare EGFR expression with hormonal status and prognostic factors, Chi-square test was applied and P < 0.05 was considered significant.
RESULTS
In our cross-sectional study of 50 breast carcinomas, the age of patients ranged from 28 to 69 years. The mean age was 49.8 ± 7.2 years. There were 44 (88%) infiltrating ductal carcinoma, 3 (6%) medullary carcinoma and 3 (6%) mucinous carcinoma.
Epidermal growth factor receptor expression was more common in females younger than 50 years (P = 0.003). Lymph node involvement was seen in 24/50 (48%) breast carcinoma patients, and there was no significant correlation between EGFR expression and lymph node involvement.
According to the molecular classification, we encountered 7 (14%) carcinomas of luminal A type, 9 (18%) of luminal B type, 15 (30%) of HER-2/neu positive type and 19 (38%) of triple-negative type.
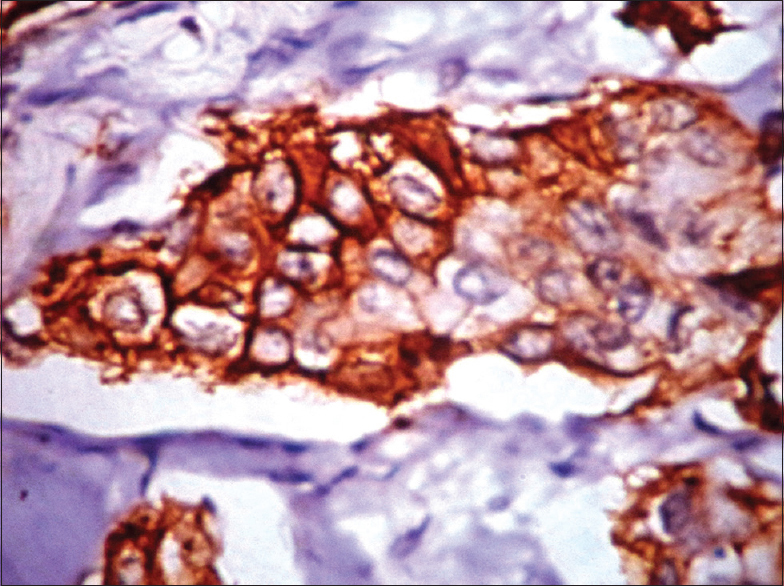
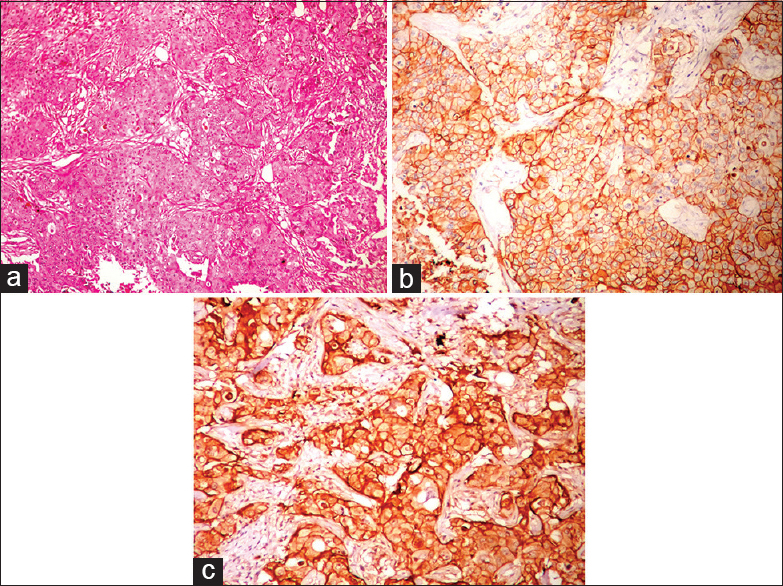
The comparison of SBR grade and EGFR expression are shown in Table 2. There was a positive correlation with Grade III and Grade II (P = 0.03491). EGFR expression was predominantly noted in 17/19 (89.47%) of triple negative type [Figure 1]. Nontriple negative tumors, 7/15 (46.67%) HER-2/neu positive carcinomas [Figure 2a–c] and 4/9 (44.44%) of luminal B (basoluminal type) carcinomas [Figure 3] also showed EGFR positivity. Out of 4 basoluminal cases, which expressed EGFR, one case (Grade II) showed 3+ positivity and other 3 were 2+. All luminal a type breast carcinomas were EGFR negative. In this study, we noted that EGFR expression was strong in triple negative tumors compared to other types.
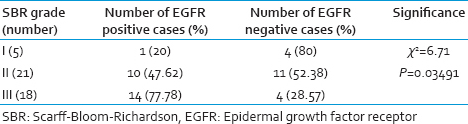
- Epidermal growth factor receptor (membrane positivity) by immunohistochemistry (IHC) in triple negative breast carcinoma (IHC, ×40)
- (a) Histopathology showing infiltrating ductal carcinoma with tumor cells in sheets (H and E, ×10); (b) human epidermal growth factor receptor (HER)-2/neu expression (membrane positivity) by immunohistochemistry (IHC) (IHC, ×40); (c) epidermal growth factor receptor expression in HER-2/neu breast carcinoma by IHC (IHC, ×40)
- Progesterone receptors expression (nuclear staining) by immunohistochemistry (IHC) in luminal B type (IHC, ×40)
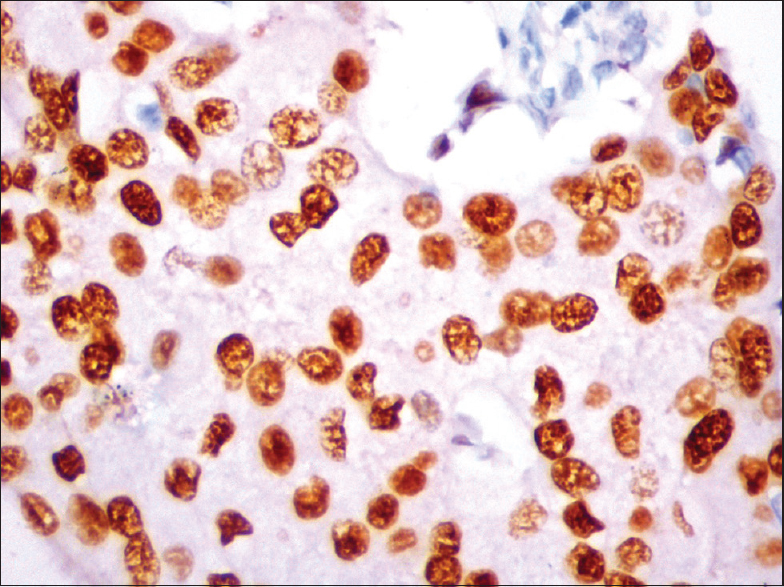
The medullary carcinomas accounted for 6% (3/50). All were triple negative and strongly expressed EGFR [Figure 4a and b].
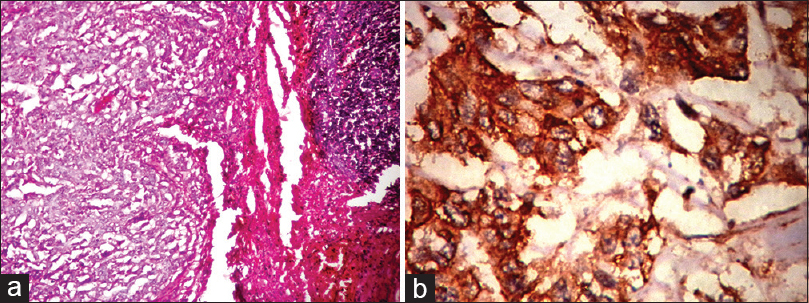
- (a) Histopathology of medullary carcinoma with large tumor cells in syncitial pattern (H and E, ×10); (b) epidermal growth factor receptor expression (membrane positivity) by immunohistochemistry (IHC) in medullary carcinoma (IHC, ×40)
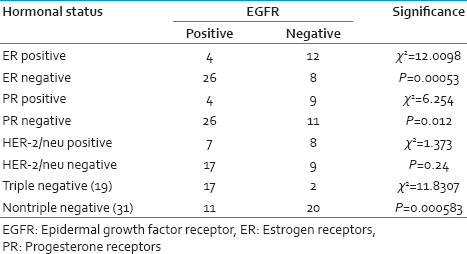
In our study, we compared EGFR expression with the hormonal status [Table 3]. There was a significant EGFR expression in tumors which were ER negative (P < 0.05). This was also similar to that seen with PR-negative tumors. There was no significant correlation between HER-2/neu and EGFR expression. The triple negative tumors very strongly expressed EGFR (P = 0.000583).
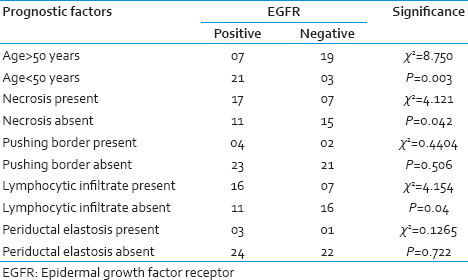
The comparison between prognostic factors and EGFR expression are shown in Table 4. EGFR expression has a strong association with the presence of necrosis and lymphocytic infiltrate. However, there was no statistical significant correlation with pushing border and periductal elastosis.
DISCUSSION
The main goal of the molecular classification was to clarify the molecular markers of prognosis and to show the potential response to adjuvant therapy in individual cases. The five molecular types of breast carcinomas were demonstrated by gene profile analysis and IHC. IHC was shown to be a good and accurate surrogate to define a particular type.[1]
Epidermal growth factor receptor plays an important role in defining basal-like carcinoma, and previous studies suggested that this receptor could be a target for specific inhibitors.[2] Basal type breast cancer is one such subtype that has been associated with EGFR expression and a poor prognosis. A majority of the “triple negative” patients have tumors of the basal subtype with EGFR expression.[6] Triple-negative tumors are the only major type of breast cancer for which no specific Food and Drug Administration-approved targeted therapy is available to improve patient outcomes; it is resistant to targeted therapies.[7]
Epidermal growth factor receptor plays a crucial role not only in the molecular diagnosis of breast cancer, but its expression also induces resistance to chemotherapy and radiation treatment, and, therefore, is a marker of poor prognosis and survival.[1] Studies have reported that EGFR is expressed in 15–45% of breast tumors and is inversely related to hormone receptor expression.[5]
The epidermal growth factor receptor expression had a strong association with young age in our study. Rimawi et al. also reported that EGFR expressing tumors occurred more often in young and minority women. They were also more likely to co-express HER-2/neu but much less likely to express hormone receptors, especially PR.[5]
The incidence of EGFR expression in triple negative tumors was very high in our study. The reason of this variation could be geographical variation.
Epidermal growth factor receptor was also expressed in nontriple negative tumors like HER-2/neu positive and Luminal B types but was negative in luminal A tumors. EGFR positive luminal carcinomas are very aggressive and are likely to have a higher risk for metastasis. The expression of EGFR by a subset of HER-2/neu type breast cancer clearly defines a subtype that is also ER-negative, and consequently associated with worst prognosis.[1]
In the recent past, the basoluminal phenotype has been described which is characterized by expression of basal markers and HER-2/neu in the luminal cells. One study has reported 2 cases of this category.[8] We have reported 4 cases of basoluminal type, however, we could not follow up these cases. These observations suggest that EGFR expression is not only limited to triple negative breast cancers, but it is also expressed in NTNBC.
The association with a different biology, such as downregulation of ER or PR was strong: 37% of EGFR positive tumors were negative for both ER and PR.[5] We reported 76.47% of EGFR positive tumors, which were negative for ER and PR. EGFR expression was inversely associated with ER status similar to the other study.[9]
The incidence of TNBC in our study was 38% and medullary carcinoma accounted for 15.79% of TNBC compared to Zhang et al., who reported 6.1% of medullary carcinoma in 15% of TNBC.[10] Many studies have demonstrated that medullary carcinoma displayed a basal-like molecular profile, similar to TNBC with poor prognosis but is associated with a relatively favorable prognosis.[211]
However, relatively few studies have tried to find an association between subtypes and prognosis in TNBC. In our study, we noted a significant association between tumor necrosis, lymphocytic infiltration, and EGFR expression, but no significant correlation with pushing border and periductal elastosis. Rao et al. reported that there was a positive correlation with necrosis and negative correlation with pushing margins and lymphocytic infiltrates.[7] Magkou et al. also reported a negative association between periductal elastosis and expression of EGFR.[9]
In our study there was significant positive association between higher grade and EGFR expression (P = 0.03491) which was similar to study by Rao et al. But unlike other studies we noted lymph node involvement in 24/50 (48%) breast carcinoma patients and there was no significant association between EGFR expression and lymph node involvement.[6]
CONCLUSION
Our study demonstrated strong EGFR expression in triple negative tumors. Its expression was also seen in a small subset of nontriple negative tumors like HER-2/neu positive and Luminal B tumors. Thus, EGFR is an important marker to stratify patients with breast cancer, according to molecular classification. The expression of EGFR correlated positively with young age, higher SBR grade, necrosis, lymphocytic infiltrate and inversely with hormonal receptor expression.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Immunohistochemical expression and significance of epidermal growth factor receptor (EGFR) in breast cancer. Rom J Morphol Embryol. 2009;50:217-21.
- [Google Scholar]
- Very low prevalence of epidermal growth factor receptor (EGFR) protein expression and gene amplification in Saudi breast cancer patients. Diagn Pathol. 2011;6:57-62.
- [Google Scholar]
- The nuclear epidermal growth factor receptor signaling network and its role in cancer. Discov Med. 2011;12:419-32.
- [Google Scholar]
- Epidermal growth factor receptor expression in breast cancer association with biologic phenotype and clinical outcomes. Cancer. 2010;116:1234-42.
- [Google Scholar]
- Immunohistochemical profile and morphology in triple – Negative breast cancers. J Clin Diagn Res. 2013;7:1361-5.
- [Google Scholar]
- Clinico-pathological characteristics of triple negative and non triple negative high grade breast carcinomas with and without basal marker (CK5/6 and EGFR) Expression at a Rural Tertiary Hospital in India. Breast Cancer (Auckl). 2012;6:21-9.
- [Google Scholar]
- Expression of the epidermal growth factor receptor (EGFR) and the phosphorylated EGFR in invasive breast carcinomas. Breast Cancer Res. 2008;10:R49.
- [Google Scholar]
- An associated classification of triple negative breast cancer: The risk of relapse and the response to chemotherapy. Int J Clin Exp Pathol. 2013;6:1380-91.
- [Google Scholar]





