Translate this page into:
Etiological profile and antimicrobial susceptibility pattern of blood culture isolates for bloodstream infection
*Corresponding author: Ekadashi Rajni, Department of Microbiology, Mahatma Gandhi Medical College and Hospital, Jaipur, Rajasthan, India. ravajni@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Purohit S, Kaur P, Lamba M, Jangid Y, Sharma C, Rajni E. Etiological profile and antimicrobial susceptibility pattern of blood culture isolates for bloodstream infection. J Lab Physicians. 2024;16:501-6. doi: 10.25259/JLP_94_2024
Abstract
Objectives:
The objective of the study is to identify and study the antimicrobial susceptibility pattern of various causative agents of bloodstream infection (BSI) in a tertiary care hospital.
Materials and Methods:
This was a retrospective observational study conducted from October 2022 to September 2023. Paired blood culture samples were collected aseptically and incubated in BD BACTEC FX-40, a fully automated blood culture system, for 5 days. When the bottle was “flagged positive,” gram staining was done, followed by subculture onto Blood agar and MacConkey agar. Species identification and antibiotic susceptibility testing were performed using the VITEK 2 Compact system and interpreted according to Clinical Laboratory Standard Institute guidelines.
Statistical analysis:
The data were analyzed using the Statistical Package for the Social Sciences version 20.0.
Results:
10,449 blood cultures were received during the 1-year study period, out of which 13.2% (1378 samples) showed growth of pathogenic organisms. Gram-negative bacilli (810/1,378, 58.8%) were the most common etiological agents, and Klebsiella pneumoniae was the most predominant pathogen recovered . Candida spp. were recovered in (150/1378, 10.9%) isolates. A high rate of antimicrobial resistance (AMR) was noted, including (26/46, 56.5%) methicillin-resistant Staphylococcus aureus, (9/125, 7.2%) vancomycin-resistant enterococci, and (269/536, 50.2%) carbapenem-resistant enterobacterales. The current study also highlights high resistance among non-fermenting Gram-negative bacteria.
Conclusions:
Gram-negative bacilli are the most common cause of BSIs in our setup. A high level of AMR is observed among the pathogens. The formulation of an effective antimicrobial stewardship program and its strict compliance is the need of the hour.
Keywords
Antimicrobial susceptibility
Antimicrobial stewardship
Bloodstream infections
Klebsiella pneumoniae
Non-fermenting Gram-negative bacteria
INTRODUCTION
Bloodstream infections (BSIs) represent a significant global burden, contributing substantially to both morbidity and mortality. They are identified by the presence of a positive blood culture in a patient exhibiting systemic signs of infection and originate either primarily or result from an established infection at another body site.[1] In critically ill patients, each hour of delay between symptom onset and the initiation of antibiotic therapy raises the mortality rate by 9%. Therefore, prompt identification of the causative agents responsible for these infections, along with their antimicrobial susceptibility profiles, is crucial for effective patient management.
Patients admitted in critical care units are particularly vulnerable to colonization by highly resistant pathogens due to factors such as overuse of broad-spectrum antibiotics, compromised immune system, presence of indwelling devices, and need for invasive procedures. An effective Antimicrobial Stewardship Program (AMSP) ensuring appropriate use of antibiotics is vital for effectively managing these infections. Baseline data about the microorganisms responsible for such infections and their antimicrobial susceptibility profile protect against the irrational use of antibiotics and aid in the prevention of antimicrobial resistance (AMR). Therefore, this study was conducted to identify the organisms responsible for BSIs in our setup and to assess the antimicrobial susceptibility patterns of the isolated strains.
MATERIALS AND METHODS
Study design
This retrospective observational study was carried out in the Department of Microbiology at Mahatma Gandhi Medical College and Hospital, Jaipur, Rajasthan, a superspecialty tertiary care teaching hospital. This center provides specialized services and caters to a wide geographical region in Rajasthan and nearby states as well as serving as an emerging center for medical tourism.
Duration
The study was conducted for 1 year, from October 2022 to September 2023.
Sample collection and processing
Paired blood culture samples were collected aseptically and incubated in the BD BACTEC FX-40, a fully automated blood culture system, for 5 days. During the incubation period, when the bottle “flagged positive,” gram staining was performed, and a critical alert was made to the clinician. Species identification and antibiotic susceptibility testing were performed using the VITEK 2 Compact system. Results were interpreted according to Clinical Laboratory Standard Institute guidelines.
Microbes presumed to be introduced during specimen collection or processing, not associated with causing illness in the patient, such as diphtheroids, Bacillus spp., Propionibacterium spp., and Micrococcus spp., were defined as contaminants. Coagulase-negative staphylococci (CoNS) were considered pathogenic only when isolated from paired blood cultures.
Statistical analysis
Descriptive statistics for categorical variables were presented as frequencies and percentages, while continuous variables were expressed as means and standard deviations. The data analysis was conducted using the Statistical Package for the Social Sciences version 20.0.
RESULTS
Ten thousand four hundred forty-nine blood samples were received in the microbiology laboratory during the study period. Among these, 1378 samples (13.2%) showed growth of pathogenic organisms, comprising 1,228 bacterial isolates (89.1%) and 150 Candida isolates (10.9%). A male-to-female ratio of 2.27:1 was observed, and the highest positivity rate was found in the 46–60 age group. The mean age of patients with positive blood cultures was 51 years, ranging from 18 to 91 years. The majority of samples were received from intensive care unit (ICU) patients (99.4%).
Figure 1 summarizes the results of the study, showing that Gram-negative bacilli were the most common isolates (810/1,378, 58.8%). The most common organism recovered was Klebsiella pneumoniae (309/1,378, 22.4%), followed by CoNS (240/1,378, 17.4%). Candida tropicalis was the most common fungal isolate (43/150, 28.7%).
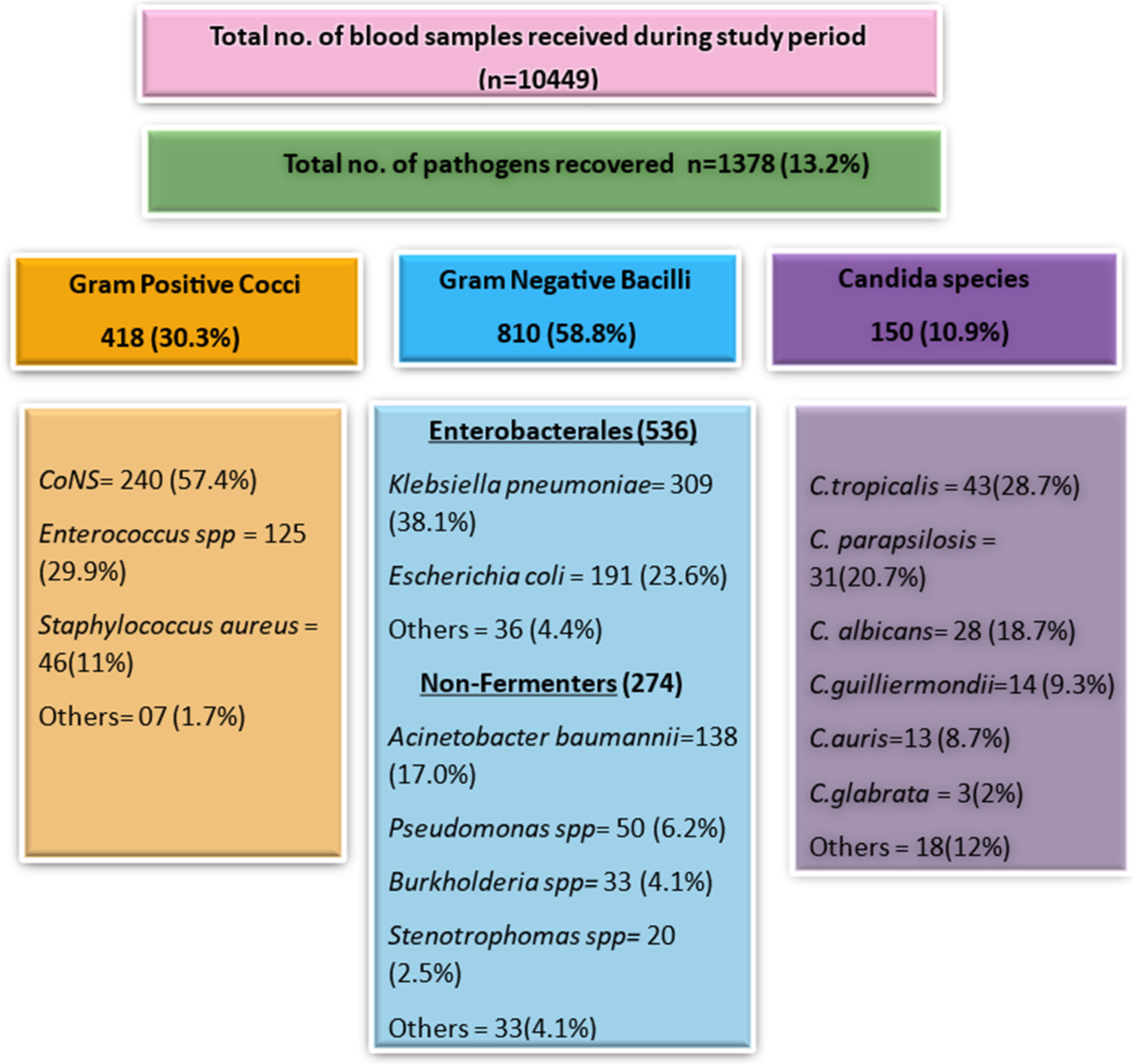
- Spectrum of most common microorganisms isolated from blood culture during the study period.
Gram-positive cocci showed the highest susceptibility to linezolid, vancomycin, and daptomycin [Table 1].
| Organism | P | OX | GEN | HLG | CIP | LE | TE | COT | E | CD | VAN | TEI | LZ | DAP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CoNS (n=240) | 0.83 | 4.16 | 49.16 | NT | 21.25 | 22.5 | 76.66 | 51.25 | 19.16 | 32.91 | 100 | 91.66 | 100 | 100 |
| Staphylococcus aureus (n=46) | 15.21 | 43.47 | 82.60 | NT | 8.69 | 15.21 | 89.13 | 80.43 | 34.78 | 67.39 | 100 | 95.65 | 100 | 100 |
| Enterococcus spp. (n=125) | 12 | NT | IR | 18.4 | 4.8 | 6.4 | 11.2 | IR | 2.4 | IR | 92.8 | 93.6 | 96.8 | 100 |
P: Penicillin, OX: Oxacillin, GEN: Gentamicin, HLG: High-level gentamicin, CIP: Ciprofloxacin, LE: Levofloxacin, TE: Tetracycline, COT: Cotrimoxazole, E: Erythromycin, CD: Clindamycin, VAN: Vancomycin, TEI: Teicoplanin, LZ: Linezolid, DAP: Daptomycin, NT: Not tested, IR: Intrinsically resistant, CoNS: Coagulase-negative staphylococci
Tables 2 and 3 present the antimicrobial and antifungal susceptibility data of the most common Gram-negative and fungal isolates, respectively.
| Organism | CAZ | CPM | ATR | MRP | IPM | P/T | CFS | GEN | AK | CIP | LE | MIN | CL (I) | COT | CXM | CTR | ETP | AMC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Klebsiella spp. (n=309) | NT | 10.35 | NT | 12.29 | 25.24 | 20.06 | 21.03 | 27.50 | 28.15 | 14.23 | NT | NT | 100 | 23.30 | 23.94 | 34.62 | 28.47 | 26.86 |
| Escherichia coli (n=191) | NT | 37.69 | NT | 57.59 | 59.68 | 39.79 | 52.87 | 62.82 | 78.53 | 3.66 | NT | NT | 100 | 38.21 | 13.61 | 29.84 | 55.49 | 24.6 |
| Acinetobacter spp. (n=138) | 6.52 | 5.07 | 5.07 | 7.97 | 5.07 | 7.24 | 15.21 | 7.97 | 7.24 | 6.52 | 6.52 | 42.02 | 100 | 26.08 | IR | IR | IR | IR |
| Pseudomonas spp. (n=50) | 44 | 42 | 0 | 36 | 40 | 28 | 40 | NT | NT | 32 | 28 | IR | 100 | IR | IR | IR | IR | IR |
| Burkholderia cepacia (n=33) | 100 | IR | IR | 69.69 | IR | IR | 18.18 | IR | IR | 24.24 | 21.21 | 69.69 | IR | 100 | NT | IR | IR | IR |
| Stenotrophomonas maltophilia (n=20) | NT | NT | IR | IR | IR | IR | NT | IR | IR | NT | 70 | 85 | IR | 75 | IR | IR | IR | IR |
CAZ: Ceftazidime, CPM: Cefepime, ATR: Aztreonam, MRP: Meropenem, IMP: Imipenem, PT: Piperacillin-tazobactam, CFS: Cefoperazone sulbactam, GEN: Gentamicin, AK: Amikacin, CIP: Ciprofloxacin, LEV: Levofloxacin, MIN: Minocycline, CL: Colistin, COT: Cotrimoxazole, CXM: Cefuroxime, CTR: Ceftriaxone, ETP: Ertapenem, AMC: Amoxicillin clavulanate, NT: Not tested, IR: Intrinsically resistant, I: Intermediate
| Organism | Fluconazole | Voriconazole | Caspofungin | Micafungin | Amphotericin B | 5-Flucytosine |
|---|---|---|---|---|---|---|
| Candida tropicalis (n=43) | 97.67 | 93.02 | 100 | 100 | 100 | 90.69 |
| Candida parapsilosis (n=31) | 35.48 | 90.32 | 93.54 | 93.54 | 93.54 | 93.54 |
| Candida albicans (n=28) | 75 | 85.71 | 92.85 | 92.85 | 78.57 | 78.57 |
A high rate of AMR was noted, including (26/46, 56.5%) methicillin-resistant Staphylococcus aureus (MRSA), (9/125, 7.2%) vancomycin-resistant enterococci (VRE), and (269/536, 50.2%) carbapenem-resistant enterobacterales (CRE).
DISCUSSION
The current study aimed to identify the spectrum of microorganisms responsible for BSIs and to analyze their antimicrobial susceptibility profiles. In the present study, blood culture positivity was detected in 13.2% of the samples, with the majority of culture-positive cases (99.4%) found among in-patients. This rate is consistent with findings from several studies conducted in India[2,3] and abroad.[4,5] Higher culture positivity was observed by Pal and Sujatha[6] and Vasudeva et al.[7] Differences in blood culture results can be linked to factors such as blood specimen volume, timing of collection, culture techniques, and variations in the epidemiology of causative agents. This study reveals a higher prevalence of BSI in males, with a male-to-female ratio of 2.27:1 and the highest positivity rate observed in the 46–60 age group. Several other studies have also documented similar results.[8]
Gram-negative BSIs were more common, a trend consistent with findings from other studies. Among Gram-negative pathogens, enterobacterales accounted for the maximum cases (66.2%) with a predominance of Klebsiella spp. (38.1%), followed by Escherichia coli (23.6%), [Figure 1] which corroborates with a study done by Banik et al.[1]
The antibiotic susceptibility pattern of common Gram-negative pathogens is summarized in Table 2. They exhibited low susceptibility to fluoroquinolones, aminoglycosides, and third-generation cephalosporins, except for E. coli, which showed moderate susceptibility to fluoroquinolones. The CRE prevalence in our study is 50.2%, which is higher than the prevalence reported by Modi et al. (29.07%).[9] Similarly, a high prevalence was observed in the study by Bajaj et al.[10] with 67.3%. These results highlight a significant rise in carbapenem resistance, likely driven by the overuse and misuse of carbapenems.
Non-fermenting Gram-negative bacteria (NFGNB) accounted for 33.8% of the isolates in our study, among which Acinetobacter spp. was the most common (50%), followed by Pseudomonas spp. (18.1%), Burkholderia cepacia (12%) and Stenotrophomonas maltophilia (7.2%) [Figure 1]. These findings are consistent with those of Manjunath et al.[11] who reported a similar isolation rate of 28.3% of NFGNB. Our findings indicated varying drug susceptibilities for B. cepacia, with trimethoprim-sulfamethoxazole and ceftazidime being 100% effective options. Similar resistance patterns were seen in studies done by Kady et al.[12] and Omar et al.[13] Drugs of choice for S. maltophilia include trimethoprimsulfamethoxazole, fluoroquinolones, and minocycline. Our findings showed 70–100% susceptibility to these drugs, consistent with the study done by Bacchani et al.[14] [Table 2]
Among 418 Gram-positive cocci, CoNS predominated (57.4%), followed by Enterococcus spp. (29.9%) and S. aureus (11%) [Figure 1]. Staphylococcus hemolyticus was the most common CoNS (47.9%) isolated, which is concordant with studies done by Gopalakrishnan and Sureshkumar[15] and Edwards et al.[16] Isolation from paired blood culture and correlation with clinical signs and biomarkers were carefully ascertained before reporting CoNS as a true pathogen. The high prevalence of CoNS in our study can be linked to our position as a tertiary care hospital that caters to a substantial population of immunocompromised patients and those with comorbidities.
MRSA prevalence in our BSI isolates is 56.5%. They are known to vary from 25% in the western part of the country to 50% in the southern regions.[17] Chaturvedi et al.[18] reported similar findings, highlighting the high prevalence rates of MRSA. In contrast, research conducted by Banik et al.[1] and Vasudeva et al.[7] indicated lower rates of MRSA. Nonetheless, MRSA remains a global threat to public health. Vancomycin is the primary treatment choice for MRSA infections. However, the emergence of vancomycin-resistant S. aureus also poses a growing concern. In the present study, S. aureus showed 100% susceptibility to vancomycin, daptomycin, and linezolid, consistent with other contemporary studies[1,3,7,18] [Table 1].
Among 224 erythromycin-resistant Staphylococcal isolates, 66% exhibited inducible clindamycin resistance (ICR), significantly higher than our previous study by Shrigaur et al. (30.6%).[19] Staphylococcus spp. also exhibited moderate susceptibility to gentamicin (82.6%), cotrimoxazole (80.4%), and tetracycline (89.1%), while showing high resistance to ciprofloxacin (91.3%), consistent with findings by Gade and Qazi[20] [Table 1].
The prevalence of Enterococcus spp. among Gram-positive bacteria was 29.9%, with Enterococcus faecium and Enterococcus faecalis comprising 85% (107/125) and 12.8% (16/125), respectively. High-level gentamycin resistance was observed in 81.6% of isolates. Susceptibility rates observed for linezolid, vancomycin, and teicoplanin were 96.8%, 92.8%, and 93.6%, respectively [Table 1]. VRE prevalence in the current study was found to be 7.2%. Over the past decade, the prevalence of VRE in India has risen, with reported rates ranging from 1% to 8.7%.[21]
Candida spp. accounted for 10.9% of BSIs in our setup, with C. tropicalis being the most common (28.7%) [Figure 1]. Globally, non-albicans Candida represents 60% of candidemia cases[22,23] and our study similarly reported 58%. Known risk factors for candidemia include prolonged health care and antibiotic exposure, ICU admission, and immunocompromised states.[24] Our isolates showed high susceptibility to echinocandins, the preferred treatment for candidemia [Table 3], consistent with findings in our previous studies.[25]
This study has a few limitations inherent to its retrospective design, with poor control over confounding factors. Furthermore, this is a single-center study, therefore data may not be generalizable. Yet, it provides vital insight into the current epidemiology of BSIs and their antimicrobial susceptibility profile. This data shall help in developing empirical therapy guidelines and providing a baseline for timely and effective management of such infections, thereby reinforcing the principles of antimicrobial stewardship.
CONCLUSIONS
BSIs are an important cause of morbidity and mortality among hospitalized patients. Gram-negative organisms have been found to be the most common cause, with K. pneumoniae being the most common isolate. A high level of AMR is observed among the pathogens, which necessitates the formulation and strict compliance of effective AMSP.
Acknowledgments
We extend our gratitude to the technicians of the microbiology laboratory for their exceptional technical support and unwavering dedication, which significantly contributed to the completion of this research.
Author’s contributions
SP: Conceptualization, data curation, formal analysis, investigation, methodology, resources, writing the original draft; PK: Data acquisition, writing the original draft, review, revising the manuscript; ML: Data analysis, editing, revising the manuscript; YJ: Data acquisition; CS: Data acquisition; ER: Design, conceptualization, data analysis and interpretation, drafting, editing, gave the final approval for publishing, overall supervision.
Ethical approval
The research/study was approved by the Institutional Review Board at Mahatma Gandhi Medical College and Hospital, number MGMC&H/IEC/JPR/2023/1730, dated September 30th, 2023.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest
Use of artificial intelligence (AI) assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for writing and editing the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Bloodstream infections and trends of antimicrobial susceptibility patterns at Port Blair. J Lab Physicians. 2018;10:332-7.
- [CrossRef] [PubMed] [Google Scholar]
- Ecology of blood stream infection and antibiotic resistance in intensive care unit at a tertiary care hospital in North India. Braz J Infect Dis. 2014;18:245-51.
- [CrossRef] [PubMed] [Google Scholar]
- Bacteriological profile and antibiogram of blood culture isolates from a tertiary care hospital of Western India. J Datta Meghe Inst Med Sci Univ. 2020;15:261-5.
- [CrossRef] [Google Scholar]
- Bacteriologic profile and antibiogram of blood culture isolates from a children's hospital in Kabul. J Coll Physicians Surg Pak. 2014;24:396-9.
- [Google Scholar]
- Bacteriological profile of neonatal septicaemia in a tertiary hospital in Nigeria. Afr Health Sci. 2006;6:151-4.
- [Google Scholar]
- Antimicrobial resistant pattern of blood culture isolates, among septicaemia suspected patients. Natl J Lab Med. 2016;5:17-21.
- [CrossRef] [Google Scholar]
- Bloodstream infections and antimicrobial susceptibility patterns in a tertiary care hospital of India. Ther Adv Infect Dis. 2016;3:119-27.
- [CrossRef] [PubMed] [Google Scholar]
- Bacterial profile, antimicrobial susceptibility patterns, and associated factors among bloodstream infection suspected patients attending Arba Minch General Hospital, Ethiopia. Sci Rep. 2021;11:15882.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of carbapenem resistant Enterobacteriaceae in a tertiary care hospital of Gujarat, India. J Clin Diagn Res. 2021;15:DC11-4.
- [CrossRef] [Google Scholar]
- Tigecycline susceptibility of carbapenem resistant Enterobacteriaceae and Acinetobacter spp. isolates from respiratory tract: A tertiary care centre study. J Krishna Inst Med Sci Univ. 2020;9:1-7.
- [CrossRef] [Google Scholar]
- Prevalence of multidrug resistant non-fermenters in a tertiary care centre. Asian J Med Sci. 2022;13:176-82.
- [CrossRef] [Google Scholar]
- Burkholderia cepacia complex among intensive care unit patients in two private hospitals in Alexandria. Int J Sci Technol Res. 2018;7:102-9.
- [Google Scholar]
- Microbiological assessment of Burkholderia cepacia complex (Bcc) isolates in Alexandria Main university hospital. Alex J Med. 2015;51:41-6.
- [CrossRef] [Google Scholar]
- Insight into Stenotrophomonas maltophilia infections in a tertiary care teaching hospital in Western India and review of literature: A retrospective observational study. J Clin Diagn Res. 2024;18:DC11-5.
- [CrossRef] [Google Scholar]
- Changing trends in antimicrobial susceptibility and hospital acquired infections over 8 years in a tertiary care hospital in relation to introduction of an infection control programme. J Assoc Physicians India. 2010;58(Suppl):25-31.
- [Google Scholar]
- Rapid and accurate identification of coagulase-negative staphylococci by real-time PCR. J Clin Microbiol. 2001;39:3047-51.
- [CrossRef] [PubMed] [Google Scholar]
- Methicillin resistant Staphylococcus aureus (MRSA) in India: Prevalence, susceptibility pattern. Indian J Med Res. 2013;137:363-9.
- [Google Scholar]
- Bloodstream infections and antibiotic susceptibility pattern in intensive care unit. Trop Doct. 2021;51:44-8.
- [CrossRef] [PubMed] [Google Scholar]
- Detection and prevalence of inducible clindamycin resistance in clinical isolates of Staphylococcus aureus Experience from a tertiary care hospital in Jaipur. Asian Pac J Health Sci. 2022;9:96-101.
- [CrossRef] [Google Scholar]
- Fluoroquinolone therapy in Staphylococcus aureus infections: Where do we stand? J Lab Physicians. 2013;5:109-12.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of vancomycin-resistant Enterococci in India between 2000 and 2022: A systematic review and meta-analysis. Antimicrob Resist Infect Control. 2023;12:79.
- [CrossRef] [PubMed] [Google Scholar]
- Candida auris A decade of understanding of an enigmatic pathogenic yeast. J Fungi (Basel). 2020;6:30.
- [CrossRef] [PubMed] [Google Scholar]
- Candida auris A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017;13:e1006290.
- [CrossRef] [PubMed] [Google Scholar]
- A Candida auris outbreak and its control in an intensive care setting. N Engl J Med. 2018;379:1322-31.
- [CrossRef] [PubMed] [Google Scholar]
- A complete clinico-epidemiological and microbiological profile of candidemia cases in a tertiary-care hospital in Western India. Antimicrob Steward Healthc Epidemiol. 2022;2:e37.
- [CrossRef] [PubMed] [Google Scholar]






