Translate this page into:
Evaluation of antibacterial properties of hydroxyapatite/bioactive glass and fluorapatite/bioactive glass nanocomposite foams as a cellular scaffold of bone tissue
Address for correspondence: Dr. Maryam Seyedmajidi, Babol University of Medical Sciences, Parastar Sq., Kargar St., Babol, Iran. E-mail: ms_majidi79@yahoo.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
AIMS AND OBJECTIVES:
Infection is a serious problem for patients after implantation surgery, which is difficult to treat with antibiotic therapy. The present study was developed to evaluate and compare the antibacterial properties of hydroxyapatite/bioactive glass (HA/BG) and fluorapatite/bioactive glass (FA/BG) nanocomposite foams as a cellular scaffold for use in bone defects by two macrodilution and disk diffusion methods.
MATERIALS AND METHODS:
Staphylococcus aureus, Enterococcus faecalis, and Streptococcus mutans were cultured in brain heart infusion broth medium with nanocomposite powder for 5 days, and their bioactivity levels were evaluated by daily culturing on solid agar medium plates. To carry out the disk diffusion test, a disc form of nanocomposite foams was used on agar medium with 48 h incubation.
RESULTS:
None of two nanocomposites even at their highest concentration (200 mg/mL) did not prevent the growth of two Staphylococcus aureus and Enterococcus faecalis microorganisms. However, HA/BG nanocomposite on the 3rd day at a concentration of 200 mg/mL and on 4th and 5th day at a concentration of 100 mg/mL and FA/BG nanocomposite on the 4th day at a concentration of 100 mg/mL and on the 5th day at a concentration of 50 mg/mL could be able to kill Streptococcus mutans microorganism. In the disc diffusion test, none of the nanocomposites could create a nongrowth zone. Both tested biomaterials showed increased antibacterial properties over time and concentration increase.
CONCLUSION:
HA/BG and FA/BG nanocomposites, due to their biocompatibility and antimicrobial properties, are good choices for implantation instead of damaged bone tissue in tissue engineering.
Keywords
Antibacterial activity
cell scaffold
nanocomposite
Introduction
Bacterial infection is a serious complication subsequent to implant surgery in orthopedics and dentistry, which can typically only be cured by removing the implant, since the biofilm mode of growth of infecting organisms on an implant surface protects the bacteria from the host immune system and antibiotic therapy.[1] Orthopedic implant infection control due to use of antibiotics in long-term period, the surgery repetition rate, and clinical and economic outcomes is difficult to apply.[23] Nowadays, the use of biocompatible synthetic materials to replace damaged tissues is considered as a necessary and unavoidable requirement.[4] Bioceramics are important subsets of biomaterials, which are employed in a variety of clinical applications, including dental materials, spinal cord repair, orthopedic applications, and drug delivery in the form of powder, coating, and bulk.[567]
Of the bioactive materials, the best bioactive behavior belongs to hydroxyapatite (HA) and bioactive glass (BG) bioceramics.[8] The combination of BG particles with HA makes special characteristics such as bioactivity and mechanical properties.[9] It was understood well that the insertion of fluoride ions into HA structure significantly increases its resistance against biodegradability and thermal decomposition.[10] If OH− groups in HA arecompletely replaced with F−, fluorapatite (FA) is formed.[11] Microorganisms have a strong tendency to cause surfaces to form a micro-ecosystem in which various microbial strains and species grow in a slime-enclosed biofilm.[31213] Antibacterial activity of bioactive ceramics is influenced by their own chemical composition and degradation conditions around them. It is reported that the antimicrobial effect of the 45S5 BG can be increased dramatically by reducing its particle size, which consequently causes the rapid release of alkaline agents.[3] It has been shown that S53P4 BG has an antibacterial effect on some oral microorganisms.[14] Furthermore, the antibacterial effects of Bioglass® on oral bacteria,[15] the use of S53P4 BG for the treatment of dental sensitivity and inflammation of the sinuses,[16] and silver-containing glass coatings in surgical sutures can also be noted.[17] Leppäranta et al. found that the antibacterial effect of bioactive ceramics depends on various factors, including high pH and osmotic effects caused by nonphysiological concentrations of ions from glass dissolution.[14]
Disk diffusion test is one of the most commonly used methods for evaluating antibacterial properties. However, the results of this test do not reflect antibacterial activity completely.[18] This test is not sensitive and semiquantitative relatively, which does not reflect the difference in bacterial growth inhibitory levels. Furthermore, the results of this test are strongly influenced by the solubility and diffusion capability of the substance in the agar.[19] To overcome these problems, it is possible to determine the bactericidal and bacteriostatic potential of materials accurately, using macrodilution method by culture of microorganisms in adjacent to different concentrations of them.
The aim of this study was to evaluate and compare the antibacterial properties of HA/BG nanocomposite foam and FA/BG nanocomposite foam as a cellular scaffold for use in bone defects in two macrodilution and disk diffusion methods.
Materials and Methods
Preparation of hydroxyapatite/bioactive glass and fluorapatite/bioactive glass nanocomposite foams
Nanosized powders of HA (Ca10[PO4]6[OH]2), FA (Ca10[PO4]6F2), and BG (58S), with chemical compositions of 58% SiO2, 36% CaO, and 6% P2O5 and particle size of <100 nm (NikCeram Razi, Isfahan, Iran), were developed through the sol–gel method and then employed as prefabricated composite foams. In addition, agarose powder (Merck, Darmstadt, Germany), Tergitol® NP-9 (Sigma-Aldrich, St. Louis, MO, USA) and sodium tripolyphosphate (STPP, Sigma-Aldrich) were used according to the gel-casting method.
For a synthesis of the HA/BG nanocomposite foam, HA and BG nanopowders (at the same weight ratio) were on sequence ground, mixed, added to 1% sodium tripolyphosphate (STPP) in deionized water, and mixed for 15 min. Then, a 7% agarose solution was added to the mixture and mixed at 130°C. Finally, 3% Tergitol was added to the suspension as the surfactant, and the foaming process was carried out by means of a 3-blade mixer at 80°C. The resulting product was poured into polyethylene molds (with the desired shape and dimensions), and gelatinization was achieved by cooling the foam at 0°C. Then, the samples were removed from the molds, dried at room temperature for 4 h, and sintered at 1200°C. For the synthesis of FA/BG nanoparticle foams, FA nanopowder was used instead of HA nanopowder. To perform the macrodilution test, the nanocomposite foams were first crushed using a mortar Muller, and their powdered form was used. The specific surface area and particle size of prepared nanocomposites were specified by Brunauer–Emmett–Teller method and transmission electron microscopy, respectively.[20]
In vitro evaluation of antibacterial activity of nanocomposites
Table 1 shows the microorganisms and their culture conditions studied in the present research. Brain heart infusion broth (BHI Broth, Sigma-Aldrich) as a liquid culture medium and BHI agar (BHI Agar, Sigma-Aldrich) for Staphylococcus aureus and Enterococcus faecalis microorganisms and blood agar (Sigma-Aldrich) containing 7.5% defibrinated sheep blood for Streptococcus mutans microorganism as solid culture media were used. To evaluate the antibacterial activity of nanocomposites, two macrodilution and disk diffusion methods were used in this study.

BHI broth was used as a liquid culture medium for macrodilution test. Macrodilution method as a bacterial sensitivity analysis was conducted according to Clinical and Laboratory Standards Institute to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the samples. The bacteria were cultured with each nanocomposite together to evaluate their antimicrobial activity. The different concentration levels of nanocomposites (200, 100, 50, 25, 12.5, 6.25, and 3.125 mg/mL) were diluted in broth in test tubes. Powders of the nanocomposites were first mixed and vortexed with the broth, and then the standard values of microorganisms (1.5 × 106 CFU/mL) were added to the tubes. First, a standard solution of 0.5 McFarland was prepared, and then a solution of bacteria with similar opacity, which contained approximately 1.5 × 108 CFU/mL, was prepared. After 100-fold dilution, a solution was made, with 1.5 × 106 CFU/mL of bacteria. Then, 1 ml of this solution was added to each tube. Bacterial culture medium without the nanocomposites and culture medium containing nanocomposites without added microorganisms were, respectively, considered as positive and negative controls.
The viability of the bacterial suspensions incubated with different concentrations of nanocomposites was assessed using solid agar plates. In this way, after 24, 48, 72, 96, and 120 h of culture of bacteria in BHI broth medium containing different amounts of nanocomposite powder, 1 μL of all test tube suspension was cultured on the agar plates using a loop. Two plates were used for each tube. The growth of the bacteria after culturing and incubation of 24 h at 37°C was evaluated by counting the number of colonies. The minimum concentration of composite that was able to inhibit the growth and increase bacteria was considered as the MIC, and the minimum concentration of composite capable of preventing the growth of 99% of the bacteria was assumed to be as MBC. The lack of observed growth of bacteria on the plate is a result of the bactericidal effect of the nanocomposite. Duplication method was used for each sample to assure the accuracy of obtained results.
In the disk diffusion test, the antibacterial activity of the composites was evaluated by examining the extent of the bacterial growth inhibitory area. At first, a 100 μL of bacterial broth medium with similar opacity to 0.5 McFarland, containing approximately 1.5 × 108 CFU/mL of bacteria, was cultured on agar medium plate using a sterile swab. Then, the nanocomposite foam discs 5 mm in length and 2 mm thickness were placed on the agar medium with 2 cm distance away from other disks and from the plate wall. After 48 h' incubation at 37°C, the bacterial inhibitory growth area was measured. For each microorganism, two plates were considered and the average results of three times replication of the experiment were reported as the final result.
To evaluate the effect of nanocomposites on the biochemical profiles of the culture medium, the powder of each nanocomposite with a ratio of 200 mg/mL in BHI broth was placed in a sterile polyethylene tube. Tubes were preserved in an incubator at 37°C for 5 days. During this period, the pH of the environment was evaluated daily with a digital pH meter. The culture medium without biomaterial was considered as control.
Results
The specific surface area for HA/BG and FA/BG was 0.40726 and 0.79761 m2/g with an average size of 78 and 42 nm, respectively. The results obtained from antimicrobial effects in different concentration are summarized in Table 2. As could be seen in the table, none of the nanocomposites even at their highest concentration (200 mg/mL) and after 5 days' incubation could not prevent the growth of two Staphylococcus aureus and Enterococcus faecalis microorganisms.
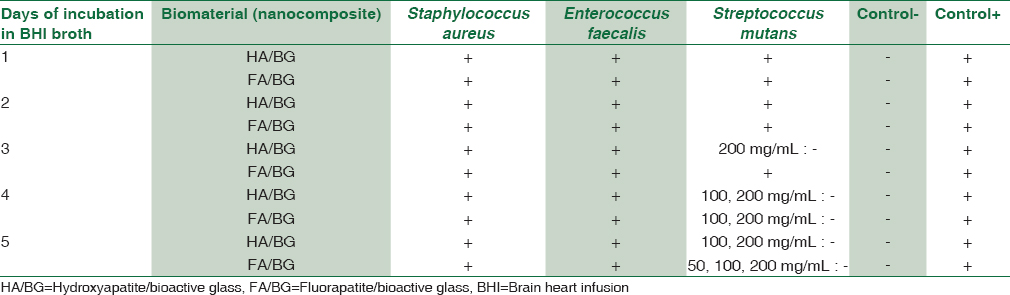
In contrast, the presence of biomaterials in the culture medium was effective in the growth of Streptococcus mutans microorganisms. After 2 days, colonies on the growth plate associated with 200 and 100 mg/mL concentrations of HA/BG were lower than the 1st day and other concentrations, but it was not so few to be countable. After 3 days, while the number of colonies on the growth plate associated with the 100 mg/mL concentration decreased, the highest concentration (200 mg/mL) was able to inhibit the growth of bacteria and killed them. The result of the antibacterial activity was repeated in the following days, and on the 4th and 5th days the lowest concentration (100 mg/mL) was achieved.
The antibacterial activity of FA/BG on the Streptococcus mutans began with a 1-day delay relative to HA/BG on the 4th day. At this time, the antibacterial activity of FA/BG began at 100 mg/mL concentration and on the 5th day progressed to a concentration of 50 mg/mL. As in all the cases the antibacterial activity of biomaterials resulted in the complete loss of microorganisms, the concentrations of 100 mg/mL for HA/BH and 50 mg/mL for FA/BG were considered as the MBC. The results of the disc diffusion test indicated that after incubation for 48 h, none of two nanocomposite disks did not prevent bacterial growth and were not be able to create a growth inhibitory zone.
The results of pH measurements of BHI medium containing 200 mg/mL biomaterials throughout 5 days of incubation are shown in Figure 1. According to the results, on the 1st day of incubation, increasing the pH resulted in alkaline condition in the culture medium.
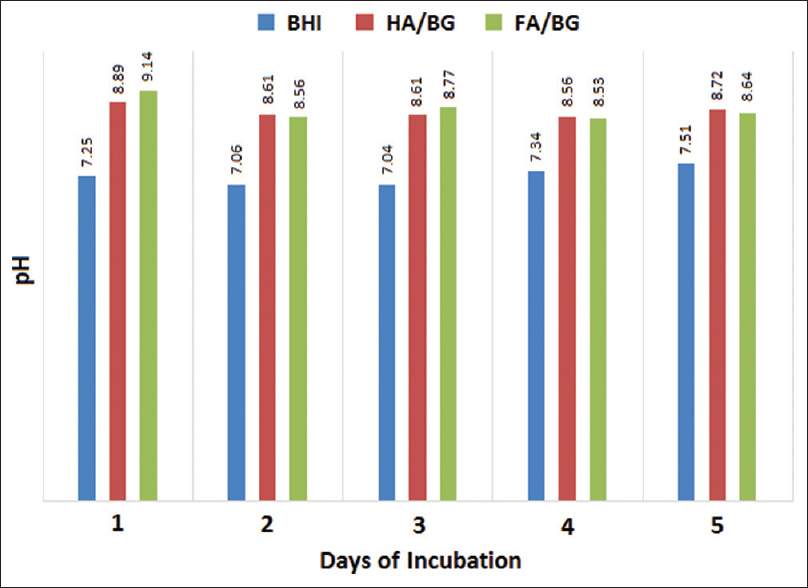
- Effects of biomaterials on brain heart infusion broth medium pH
Discussion
In this study, we investigated the antibacterial properties and determined the MIC and MBC of two nanocomposites made, as bone tissue scaffolds. Three of the most important bacteria in the development of bone infections were cultured at seven different concentration levels of biomaterials in broth medium for 5 days, and the antibacterial effects of biomaterials were evaluated daily by culture on agar medium. None of the two biomaterials at maximum concentration could affect the growth of Staphylococcus aureus and Enterococcus faecalis microorganisms. However, from 3rd to 4th days, HA/BG and FA/BG in respect were able to inhibit the growth of Streptococcus mutans microorganisms.
In the study conducted by Munukka et al., after 6 days of antimicrobial susceptibility testing and at the highest concentration (100 mg/mL), E. faecalis was the most resistant bacteria to the various BGs.[21]
Mortazavi et al. studied the antibacterial properties of BG nanoparticles made by the sol–gel method on aerobic bacteria and did not show antibacterial properties at concentrations below 50 mg/mL after 5 days of culture. However, 58S and 63S BG were able to eliminate Staphylococcus aureus completely at concentrations of 50 and 100 mg/mL, respectively. The initial pH of medium was 7.3, but after incubation for 24 h at concentrations of 50 and 100 mg/mL, it was 8.3 and 8.8, respectively, and after 120 h it reached at 9. These findings suggest that pH is a limiting factor in the growth of microorganisms.[3]
In this study, both two tested biomaterials biomaterials showed higher inhibitory effects with increasing of biomaterials concentration and incubation time. Ragab et al., while studying the antibacterial activity of HA nanoparticles, concluded that bacterial growth decreased rapidly with increasing HA nanoparticle concentration.[22]
The initial powders of the nanocomposites used in this study were prepared by the sol–gel method. This method is simple and produces particles of very small size in nanoscale. Particle size affects their antibacterial properties. In fact, by decreasing the particle size and thus increasing the surface area, contact with the surrounding environment will increase, resulting in increased solubility. Therefore, a nanosized amorphous structure could have an optimal antibacterial effect due to faster ion release than structures with common grain size.[23] In addition, factors such as production of active oxygen species, due to the presence of HA, electrostatic interaction between HA nanoparticles and the cell wall, and the penetration of the HA nanoparticles into the cell and the reformation of HA in the cell can be prevented by cell wall formation and growth of bacteria.[24252627] As a result, the antibacterial potency of biomaterials can be different according to the characteristics of different species of bacteria, especially their structural properties.[2728] Furthermore, the characteristics of the bacteria cell wall such as wall permeability and the ability to dissolve HA into the cell can be considered.[27] Creating particles with a wide range of nanoscale sizes can also be another factor influencing the antibacterial properties, which is provided in this study. The pore space between particles mediates the penetration of environment in the structure and causes particles to decompose faster. Such a structure will also have a better contact with organic components in the in vivo environment.[3]
According to simulated body fluid biochemical profile changes, in result of immersion of HA/BG and FA/BG at 37°C in our previous study[20], formation of the hydroxycarbonate apatite layer (natural apatite) on the surface of the biomaterials indicates their biocompatibility. By increasing the immersion time the concentration of calcium increases due to its higher release rate than condensation. In contrast, the concentration of phosphorus decreases, which is reported by other researchers.[29303132] Silicate ions, which are in the structure of BG, will release over time. This process involves the displacement of ions, by altering and increasing the osmotic pressure and pH of the environment, which ultimately disrupts the conditions for the growth of bacteria and induces the antimicrobial activity of these biomaterials.[21333435] Another factor can be the disturbance of the bacterial membrane potential due to high concentrations of calcium and other alkaline agents released from biomaterials.[21] It has already been shown that the importing of fluoride ions into the structure of HA significantly increases the resistance of it to biodegradability and thermal decomposition. Therefore, it can be concluded that decomposition and biological effects rate of FA/BG begin later than HA/BG.[10] In our study, the antimicrobial effect of FA/BG on Streptococcus mutans was observed with 1-day delay and high intensity (beginning at 100 mg/mL concentration) compared to HA/BG. This effect could be due to the presence and gradual release of fluorine ions from FA/BG, which is an active oxidative agent and can easily react.
Conclusion
Both two examined biomaterials in this study had an almost identical antibacterial effect only on the Streptococcus mutans and showed an increasing trend in antibacterial properties over increasing concentrations and time of incubation and have an equal ratio of apatite and BG, which can result in different physical and biological properties by altering these proportions and changing the amount of polymer basic material or sintering temperature. The HA/BG and FA/BG nanocomposite foams, due to the ability to produce antimicrobial properties and their proper biocompatibility, are good choices for use in tissue engineering as cell scaffolds in damaged bone tissue.
Financial support and sponsorship
Babol University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors gratefully acknowledge the financial support for this work by Babol University of Medical Sciences. This was an approved research proposal in Babol University of Medical Sciences # 9441219.
References
- Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422-9.
- [Google Scholar]
- Antibacterial effects of sol-gel-derived bioactive glass nanoparticle on aerobic bacteria. J Biomed Mater Res A. 2010;94:160-8.
- [Google Scholar]
- Dental applications of nanostructured bioactive glass and its composites. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5:399-410.
- [Google Scholar]
- Synthesis and characterization of fluorapatite-zirconia composite nanopowders. Ceram Int. 2013;39:4329-37.
- [Google Scholar]
- Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:234-48.
- [Google Scholar]
- Development of bioactive and biodegradable chitosan-based injectable systems containing bioactive glass nanoparticles. Acta Biomater. 2009;5:115-23.
- [Google Scholar]
- In vivo evaluation of bioactive nano bioceramics for bone regeneration and repair: An animal study. Iran J Orthop Surg. 2012;39:67-76.
- [Google Scholar]
- Preparation and characterization of hydroxyapatite-forsterite-bioactive glass nanocomposite coatings for biomedical applications. Ceram Int. 2012;38:1325-30.
- [Google Scholar]
- Mechanochemical synthesis of fluorapatite-zinc oxide (FAp-ZnO) composite nanopowders. ISRN Ceram 2012 2012:9.
- [Google Scholar]
- Histopathological, histomorphometrical, and radiological evaluations of hydroxyapatite/bioactive glass and fluorapatite/bioactive glass nanocomposite foams as cell scaffolds in rat tibia: An in vivo study. Biomed Mater. 2018;13:025015.
- [Google Scholar]
- Orthopaedic-implant infections by Escherichia coli: Molecular and phenotypic analysis of the causative strains. J Infect. 2012;64:169-75.
- [Google Scholar]
- Outcome of acute prosthetic joint infections due to gram-negative bacilli treated with open debridement and retention of the prosthesis. Antimicrob Agents Chemother. 2009;53:4772-7.
- [Google Scholar]
- Antibacterial effect of bioactive glasses on clinically important anaerobic bacteria in vitro. J Mater Sci Mater Med. 2008;19:547-51.
- [Google Scholar]
- Antibacterial activity of particulate bioglass against supra- and subgingival bacteria. Biomaterials. 2001;22:1683-7.
- [Google Scholar]
- Mineralization of dentin induced by treatment with bioactive glass S53P4 in vitro. Acta Odontol Scand. 2004;62:14-20.
- [Google Scholar]
- In vitro attachment of Staphylococcus epidermidis to surgical sutures with and without Ag-containing bioactive glass coating. J Biomater Appl. 2004;19:47-57.
- [Google Scholar]
- In vitro evaluation of the antimicrobial efficacy of four endodontic biomaterials against Enterococcus faecalis, Candida albicans, and Staphylococcus aureus. Int J Biomater 2014 2014:383756.
- [Google Scholar]
- Evaluation of antimicrobial effect of root canal sealers. Pak Oral Dent J. 2011;31:432-5.
- [Google Scholar]
- Synthesis and Characterization of Hydroxyapatite/Bioactive Glass Nanocomposite Foam and Fluorapatite/Bioactive Glass Nanocomposite Foam by Gel Casting Method as Cell Scaffold for Bone Tissue. Eurasian J Anal Chem. 2018;13(3):em17.
- [Google Scholar]
- Bactericidal effects of bioactive glasses on clinically important aerobic bacteria. J Mater Sci Mater Med. 2008;19:27-32.
- [Google Scholar]
- Synthesis and in vitro antibacterial properties of hydroxyapatite nanoparticles. IOSR J Pharm Biol Sci. 2014;9:77-85.
- [Google Scholar]
- Antimicrobial effect of nanometric bioactive glass 45S5. J Dent Res. 2007;86:754-7.
- [Google Scholar]
- Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013;9:7591-621.
- [Google Scholar]
- A hydrothermal synthesis of eggshell and fruit waste extract to produce nanosized hydroxyapatite. Ceram Int. 2013;39:8183-8.
- [Google Scholar]
- Rod-like micelle templated synthesis of porous hydroxyapatite. Ceram Int. 2013;39:8995-9002.
- [Google Scholar]
- Preparation and characterization of nanostructured hydroxyapatite using a biomaterial. Synth Reactivity Inorg Metal Organic Nano Metal Chem. 2011;41:513-6.
- [Google Scholar]
- Preparation and characterization of isotactic polypropylene reinforced with hydroxyapatite nanorods. J Macromol Sci B. 2011;50:1983-95.
- [Google Scholar]
- Analysis of pore interconnectivity in bioactive glass foams using X-ray microtomography. Scr Mater. 2004;51:1029-33.
- [Google Scholar]
- Mechanical alloying synthesis and bioactivity evaluation of nanocrystalline fluoridated hydroxyapatite. J Cryst Growth. 2009;311:1392-403.
- [Google Scholar]
- Effect of the composition of hydroxyapatite/bioactive glass nanocomposite foams on their bioactivity and mechanical properties. Mater Res Bull. 2012;47:3523-32.
- [Google Scholar]
- Do bioactive glasses convey a disinfecting mechanism beyond a mere increase in pH? Int Endod J. 2008;41:670-8.
- [Google Scholar]
- Dentin enhances the effectiveness of bioactive glass S53P4 against a strain of Enterococcus faecalis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:530-5.
- [Google Scholar]
- Dissolution, leaching, and Al2O3 enrichment at the surface of bioactive glasses studied by solution analysis. J Biomed Mater Res. 1993;27:941-8.
- [Google Scholar]





