Translate this page into:
Evaluation of Nordmann, Dortet, and Poirel test for the identification of extended spectrum betalactamase production among urinary isolates of Escherichia coli
Address for correspondence: Dr. R. Deepa, N902, The Metrozone, 44, Pillayar Koil Street, J. N. Road, Annanagar, Chennai - 600 040, Tamil Nadu, India. E-mail: deeparsn@yahoo.co.in
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
CONTEXT:
Current phenotypic techniques for extended spectrum beta lactamase (ESBL) detection can be interpreted after 24 h of incubation only, resulting in a delay in initiating therapy. Nordmann, Dortet, and Poirel (NDP) in 2012 proposed a novel test named ESBL NDP to overcome this limitation.
AIMS:
This study aimed to evaluate the ESBL NDP test for the identification of ESBL among Escherichia coli isolates against the Clinical Laboratory Standards Institute-recommended phenotypic confirmatory method.
SETTINGS AND DESIGN:
This cross-sectional study was conducted over a period of 3 months on a sample size of 100.
SUBJECTS AND METHODS:
One hundred nonduplicate clinically significant E. coli urinary isolates positive by initial screening test for ESBL were subjected to the ESBL NDP test and phenotypic confirmatory test. The NDP test was evaluated by determining the sensitivity, specificity, kappa value, and confidence interval (CI) for kappa.
RESULTS:
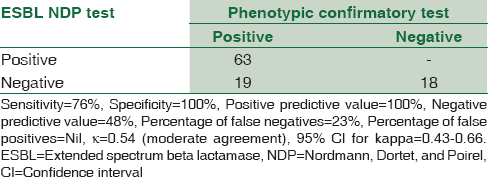
The phenotypic confirmatory test and the ESBL NDP test were positive in 82% and 63% of the isolates, respectively. ESBL NDP test had a sensitivity and specificity of 76% and 100%, positive and negative predictive values of 100% and 48%, respectively, kappa value of 0.54 (moderate agreement), and 95% CI for kappa of 0.43–0.66. The time to positivity was 1 h in 93.6% of the isolates.
CONCLUSION:
The NDP test showed a good specificity, with time to positivity of 1 h. The low sensitivity could be due to the difference in the phenotypic type of ESBL producer and technical reasons.
Keywords
Extended spectrum beta lactamase
Nordmann
Dortet
and Poirel test
phenotypic confirmatory test
Introduction
Extended spectrum beta lactamase (ESBL) is a beta lactamase which may confer resistance or reduced susceptibility to the oxy-iminocephalosporins (cefotaxime [CTX], ceftriaxone, ceftazidime) and monobactams (e.g., aztreonam). However, ESBLs do not hydrolyze the cephamycins (e.g., cefoxitin and cefotetan) and the carbapenems (imipenem, meropenem) and their hydrolytic activity can be inhibited by beta lactamase inhibitors such as clavulanic acid and tazobactam.[12]
Urinary tract infection is a major source of bacteremia, the most common causative agent being Escherichia coli. ESBL production among E. coli has become endemic in health care-associated and community-acquired infections.[3]
Molecular characterization of E. coli isolates in Indian literature shows that the majority of the ESBLs possessed the CTX-M gene, followed by TEM and SHV genes.[45]
Current techniques for ESBL detection recommended by the Clinical Laboratory Standards Institute (CLSI) are based on the determination of susceptibility to extended spectrum cephalosporins followed by the inhibition of ESBL activity by the use of beta lactamase inhibitors such as clavulanic acid (CA) or tazobactam.[6] These test results are interpreted after 24 h of incubation, resulting in a delay in initiating therapy. Molecular testing of the isolates is costly and requires considerable expertise. With these limitations in mind, Nordmann, Dortet, and Poirel (NDP) in 2012 proposed a novel test named ESBL NDP test which reportedly gave rapid and accurate identification of ESBL-producing isolates.[78]
The aims and objectives of the study were to isolate and screen clinically significant E. coli from urine samples for ESBL production and to evaluate the ESBL NDP test against the CLSI-recommended phenotypic confirmatory test so as to determine whether it can be incorporated in the routine diagnostic laboratory.
Subjects and Methods
This prospective cross-sectional study was conducted over a period of 3 months from August to October 2015. Approval from the Institutional Ethics Committee was obtained.
Sample processing and initial screen test
The semi-quantitative culture of clean-catch mid-stream urine samples was performed, and colony count was determined. Lactose-fermenting colonies with a colony count of >105 cfu/ml were subjected to standard biochemical reactions. One hundred E. coli isolates were subjected to initial screen test for ESBL production by disc diffusion test using cefotaxime (CTX) 30 μg and cefpodoxime 30 μg on Mueller-Hinton agar. Isolates with a zone of inhibition of ≤26 mm and ≤22 mm were presumptively identified as ESBL producers.[69]
Phenotypic confirmatory test for extended spectrum beta lactamase production
One hundred presumptive ESBL-producing E. coli isolates were tested initially using CTX (30 ug) and ceftazidime (CAZ) (30 μg) discs alone and in combination with CA (10 μg). The test was considered positive when an increase in the growth inhibitory zone around either the CTX or the CAZ disc with CA was 5 mm or greater of the diameter around the disc containing CTX or CAZ alone. ATCC E. coli 25922 and Klebsiella pneumoniae ATCC 700603 were included as controls.[6] The phenotypic confirmation test was chosen as it is recommended by the CLSI for confirming ESBL production and due to its ease of performance in the routine diagnostic setting.
Extended spectrum beta lactamase-Nordmann, Dortet, and Poirel test
The E. coli isolate was inoculated onto Mueller–Hinton agar and incubated at 37°C for 16–24 h before the ESBL NDP test was performed. This isolate was resuspended in 150 μl of 20 mM Tris-HCl lysis buffer distributed in sterile Eppendorf tubes with sterile glass beads. Mechanical lysis of bacteria was performed by agitation using a vortex adaptor for 30 min at room temperature. This bacterial suspension was centrifuged at 10,000 ×g at room temperature for 5 min. A 30 μl volume of the supernatant was mixed in a well of a 96-well microtiter tray with 100 μl of a 1-ml solution made of 3 mg of purified cefotaxime sodium salt (HiMedia, India) in a pH 7.8 phenol red solution. Similarly, culture extracts were analyzed in wells containing cefotaxime and tazobactam (4 mg/ml) (Sterile India, New Delhi, India) followed by 15 min incubation. A control well with only the indicator dye was included. The test result was interpreted as positive when the well containing cefotaxime alone turned from red to yellow/orange and the well containing cefotaxime supplemented with tazobactam remained red (unchanged color) [Figure 1]. The wells were observed at 30 min, 1 h, and 2 h. Change of color up to 2 h was interpreted as positive.[7]

- Microtiter wells showing positive and negative extended spectrum beta lactamase Nordmann, Dortet, and Poirel test
Results
The initial screen test for presumptive identification of ESBL production was positive in 49.7% of the E. coli isolates. One hundred isolates of E. coli which were positive by initial screen test for ESBL production were subjected to phenotypic confirmatory tests for ESBL and NDP tests. Eighty-three isolates (83%) were positive by the phenotypic confirmatory test.
Among the 100 isolates, 63 isolates (63%) were positive by the NDP test. There was no change of color immediately at the end of the 15 min incubation. A positive test was observed in 59 isolates (93.6%) in 45 min to 1 h after the completion of incubation. At 2 h, color change was observed in all the 63 isolates [Table 1].

Considering the phenotypic confirmatory test as the gold standard, the ESBL NDP test was evaluated. The sensitivity and specificity of the test were 76% and 100%, respectively, with 23% false negatives. Kappa statistics[10] showed a moderate agreement with the confirmatory test (kappa value: 0.54) (95% confidence interval for kappa: 0.43–0.66) [Table 2].
Discussion
ESBL production which mediates resistance to beta lactam antibiotics is a major issue with members of the Enterobacteriaceae family. Increasing the laboratory capability to detect ESBL production is one of the important efforts to control these organisms. This is necessary to prevent horizontal transfer of the resistant strains and treatment failure.[911] The CLSI guidelines opine that it is no longer necessary to perform routine ESBL testing; however, it may still be useful for epidemiological and infection control purposes. In India, the importance of infection control activities is being recognized and increasingly promoted. ESBL screening is one of the important surveillance tools in an infection control program.
The ESBL NDP test has been validated on cultured bacteria as well as directly on urine samples. The advantages of the test are reported to be its rapidity, high sensitivity, and specificity.[7] The intention of the study was to evaluate the ESBL NDP test and to determine whether this test can replace the phenotypic confirmatory test in the microbiology laboratory attached to a government tertiary care institution receiving approximately 200 samples/day for bacterial culture.
E. coli is the leading cause of urinary tract infection, with ESBL-producing isolates in two-thirds of cases.[3] In this study, the frequency of ESBL-producing E. coli was 82%. The NDP test was evaluated by considering phenotypic susceptibility test as the gold standard. The results were compared with the original study of Nordmann et al. The specificity and sensitivity of the NDP test were 100% and 76%, respectively. The original study by Nordmann et al. reported a specificity and sensitivity of 100% and 92.6%, respectively.[7] The test had an excellent sensitivity (100%) for CTX-M producers and was less sensitive (88%) for non-CTX-M producers.[712] The ESBL NDP test could give a negative result in the presence of a carbapenemase with metallo-lactamase as well as with a carbapenemase of the OXA-48 type.[46] Molecular methods to determine the ESBL type of the strains positive by phenotypic method but negative by ESBL NDP test were not done and it is one of the lacunae of our study. A major technical difference from the original study which could have impacted the sensitivity is the source and potency of the antibiotic powder. Other causes are weak hydrolysis of cefotaxime or due to low-level production of the ESBL.
Similar studies on the performance of the ESBL NDP test in Indian scenario were not found. However, foreign studies have compared the ESBL NDP test with flow cytometry and disc diffusion method[13] which reported a sensitivity of 100% but did not include well-characterized strains of ESBL producers. Renvoisé et al., while studying the performance of a commercial test, the β-LACTA test (Bio-Rad, Marnes-la-Coquette, France), based on the cleavage of a chromogenic cephalosporin, observed that the ESBL NDP test was more laborious and time consuming than the former.[14] However, Poirel et al. reported a better specificity (100%) and sensitivity (95%) of ESBL NDP test than commercial tests such as the β-LACTA test and the Rapid ESBL Screen kit 98022 (Rosco-Diagnostica A/S, Taastrup, Denmark) using a collection of strains possessing well-characterized resistance mechanisms.[12]
The turnaround time in our study was 1 h in 93.6% of the isolates. There was no further change of color beyond 2 h. According to the original study by Nordmann et al., interpretable results were obtained for all CTX-M producers at the end of 15 min of incubation with a maximum time limit of 30 min when the test was performed on bacterial cultures. However, while performing the test on spiked blood cultures, the total time required to obtain results was 2 h.[7]
Nordmann et al. observed that a positive cefotaxime hydrolysis result was always associated with the expression of either an ESBL or a plasmid-mediated cephalosporinase and high minimum inhibitory concentration values for cefotaxime that were above the resistance breakpoints. A negative result could not exclude the possibility of the presence of a broad-spectrum cephalosporin-resistant strain resulting from porin deficiency associated with low-level beta-lactamase activity. In case of ESBL-positive and ampicillin C (AmpC)-overproducing isolates, corresponding AmpC could hydrolyze cefotaxime at high level leading to a false-negative result.[78]
The test has the advantage that multiple samples can be tested at the same time in the microtiter well format and the results can be interpreted on the same day. The requirement of microtiter pipettes, pipette tips, tubes with microbead, vortex adaptor for continuous vortexing for 30 min, a high-speed centrifuge for centrifugation at 10,000 rpm, and antibiotic powders (cefotaxime and tazobactam sodium salt) is a disadvantage. It is also important that the antibiotic powders are stored at the appropriate temperature as this could also affect the sensitivity of the test.
In contrast, though the phenotypic confirmatory test recommended by the CLSI requires 24 h incubation, it does not require special equipment and consumables other than the incubator, Petri dishes, and Mueller–Hinton agar medium which are generally available in most of the diagnostic laboratories performing microbiological investigations. The antibiotic discs used for the test are easily available and are more stable on storage at 2°C–8°C for longer period. No specific training is required as the technologists are familiar with disc diffusion method.
Conclusion
The novel rapid NDP test has an excellent specificity with a short time to positivity of 1 h. As the test yielded a lower sensitivity in our study, in contrast to the results of Nordmann et al., possibly due to combination of factors such as the phenotypic type of ESBL producer and technical reasons, the repeatability and feasibility of the test in the routine diagnostic laboratory with limited resources need to be further evaluated.
Financial support and sponsorship
This study was financially supported by the Indian Council of Medical Research fund for STS project.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This research was carried out under the STS program of the Indian Council of Medical Research which supports the research of undergraduate medical students.
References
- Extended-spectrum beta-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14:933-51.
- [Google Scholar]
- Extended-spectrum-lactamases: A clinical update. Clinical microbiology reviews. 2005;18:657-86.
- [Google Scholar]
- Clinico-microbiological profile of urinary tract infection in South India. Indian J Nephrol. 2011;21:30-6.
- [Google Scholar]
- Epidemiology and outcome of bacteremia caused by extended spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella spp. in a tertiary care teaching hospital in South India. J Assoc Physicians India. 2010;58(Suppl):13-7.
- [Google Scholar]
- Prevalence and antibiogram of extended spectrum ß-lactamase (ESBL) producing Gram negative bacilli and further molecular characterization of ESBL producing Escherichia coli and Klebsiella spp. J Clin Diagn Res. 2013;7:2173-7.
- [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Fourth Informational Supplement. In: CLSI Document M100-S24. Wayne PA: Clinical and Laboratory Standards Institute; 2014.
- [Google Scholar]
- Rapid detection of extended-spectrum-ß-lactamase-producing Enterobacteriaceae. J Clin Microbiol. 2012;50:3016-22.
- [Google Scholar]
- Rapid detection of extended-spectrum-ß-lactamase-producing Enterobacteriaceae from urine samples by use of the ESBL NDP test. J Clin Microbiol. 2014;52:3701-6.
- [Google Scholar]
- Bailey and Scott's Diagnostic Microbiology (13th ed). St. Louis, Missouri: Elsevier; 2007. p. :926-9.
- Escherichia, Salmonella and Shigella. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, eds. Manual of Clinical Microbiology Vol Vol. 1. (9th ed). Washington, DC: ASM Press; 2007. p. :670-87. Ch. 43
- [Google Scholar]
- Comparison of three biochemical tests for rapid detection of extended-spectrum-β-lactamase-producing Enterobacteriaceae. J Clin Microbiol. 2016;54:423-7.
- [Google Scholar]
- Detection of extended-spectrum beta-lactamases (ESBLs) in clinical isolates of Klebsiella pneumoniae using the ESBL NDP test and flow cytometric assay in comparison to the standard disc diffusion. African Journal of Microbiology Research. 2016;10:238-44.
- [Google Scholar]
- Evaluation of the ßLacta test, a rapid test detecting resistance to third-generation cephalosporins in clinical strains of Enterobacteriaceae. J Clin Microbiol. 2013;51:4012-7.
- [Google Scholar]





