Translate this page into:
Evaluation of Rapid SARS-CoV-2 Antigen Detection as a Single Diagnostic Test and When Combined with C-Reactive Protein Level or Neutrophil to Lymphocyte Ratio in Suspected COVID-19 Subjects
Address for correspondence: Mervat Mashaly, MD, Department of Clinical Pathology, Clinical Microbiology Unit, Faculty of Medicine, Mansoura University, Mansoura City 35511, Egypt (e-mail: mervatmashaly@yahoo.com; mervatmashaly@mans.edu.eg).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Rapid antigen detection tests of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) play a crucial role in the control of the current coronavirus disease 2019 (COVID-19) pandemic. Data about the real diagnostic performance of such tests is still insufficient and hence their evaluation is of high priority.
Objectives
The aim of this study was to evaluate the diagnostic performance of BIOCREDIT COVID-19 antigen test alone and in combination with either C-reactive protein (CRP) or neutrophil/lymphocyte ratio (NLR) in comparison to real-time quantitative polymerase chain reaction (RT-qPCR). Additionally, we investigated the selection criteria of the suspect for best performance of the antigen test.
Materials and Methods
Paired nasopharyngeal (NP) swabs were collected from 200 suspected COVID-19 subjects for SARS-CoV-2 RNA by RT-qPCR and for antigen detection by BIOCREDIT test. Simultaneously, for all suspect, clinical presentations were recorded as well as CRP level and NLR were determined.
Results
Among 200 tested NP swabs, 125 (62.5%) were RT-PCR positive. Overall sensitivity, specificity and accuracy of BIOCREDIT test were 34.4, 98.7, and 58.5%, respectively. Sensitivity of the BIOCREDIT test was higher in COVID-19 suspect, with high viral load (100%), severely ill (56.2%), with fever alone (40%), elevated CRP (41.1%), and high NLR (36.2%). In combination with NLR or CRP, sensitivity of BIOCREDIT test increased to 89.4 and 81.6%, respectively, while its specificity decreased to 67 and 59%, respectively.
Conclusion
The overall low sensitivity of BIOCREDIT/COVID-19 antigen test does not permit its use as a single diagnostic test for COVID-19. However, its use should be restricted only if it is combined with either CRP or NLR in suspect with certain criteria.
Keywords
antigen
CRP
SARS-CoV-2
lateral flow immunochromatography
RT-PCR
Introduction
Coronavirus disease-19 (COVID-19) is a recently discovered pneumonia caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at the end of 2019. At first, it was recorded in Wuhan, China.[1] Thereafter, it spread quickly to other regions in China and all over the world.[2] On March 11, 2020, the World Health Organization (WHO) stated that COVID-19 became a global pandemic.[3]
Central to controlling the rapid spread of this pandemic is the accurate and rapid identification of COVID-19 patients who should be isolated and managed at once according to stringent measures of infection control. Currently, the gold standard and recommended laboratory method for diagnosis of the COVID-19 is the real-time reverse-transcription polymerase chain reaction (rRT-PCR) for the detection of SARS-CoV-2 RNA in respiratory tract specimens.[4]
In the same context, it has shown that lymphocytopenia considers a common finding among COVID-19 patients. Consequently, the neutrophil to lymphocyte ratio (NLR) in peripheral blood has been proposed to be helpful in COVID-19 diagnosis. Additionally, C-reactive protein (CRP) has been shown to be elevated in COVID-19 patients.[5]
Although PCR assays are sensitive and specific in detection of COVID-19, they are slow, of high cost, and need professional well-trained laboratory staff as well as specialized laboratories and equipments. Thus, they are not suitable for use on a wide-scale population.[6] Therefore, there is a strong need for fast and easy-to-perform tests, especially in regions with inadequate access to molecular diagnostics. As of August 18, 2020, the Foundation for Innovative New Diagnostics (FIND) listed 18 SARS-CoV-2 rapid antigen-detection (RAD) tests and 163 rapid SARS-CoV-2 antibody detection tests that are currently marketed or in development, of which, respectively, 17 and 155 have regulatory approval by the European Community (Conformité Européenne [CE] mark).[7]
Independent research studies found that sensitivities of these newly developed RAD tests are often different from those reported by their manufacturers.[8] WHO recommended full evaluation of the diagnostic performance of these rapid tests before its clinical use during the pandemic.[9]
Therefore, the present study aimed to evaluate the diagnostic performance of BIOCREDIT/COVID-19 antigen test (one of the commercially available rapid SARS-CoV-2 antigen detection test) in suspected COVID-19 subjects when used alone and in combination with either CRP or NLR. Also, we performed a subgroup analysis to evaluate the differential effect of clinical presentation and routine laboratory tests of COVID-19 suspect on the diagnostic performance of this rapid antigen test.
Materials and Methods
Sample and Data Collection
This cross-sectional study was conducted on 200 participants selected from 1877 COVID-19 suspected subjects who referred to the quarantine department of Mansoura University Hospital in the period from January 1 to March 31, 2021. Suspicion of COVID-19 infection was based on clinical symptoms suggestive of COVID-19 and/or recent contact with a PCR-confirmed patient within 2 weeks prior to inclusion in the study.[10] All participants were categorized into subgroups according to their clinical symptoms and severity, viral load, CRP level, and NLR.
From all participants, two nasopharyngeal (NP) swabs were obtained by well-trained healthcare personnel. One swab was used for RT-PCR, obtained by Dacron polyester swabs and placed in viral transport medium (Bioscience, free zone, Ismalia, Egypt). The other swab used for antigen detection was taken and placed immediately in assay diluent tube provided with the RAD kit. Swabs were transported immediately at 2 to 8 °C to the biosafety level-2 (BSL-2) designated laboratory in our hospital. Infection control measures were implemented during sampling and transport of the swabs. This study was a single-blinded study, after performing PCR analysis, each sample was given a specific number code, and then samples were tested blindly by the antigen kit, the number code was not disclosed until the end of the evaluation.
All samples were kept at 4 °C until processed in the same day of the receipt. Simultaneously, 5 mL venous blood, (3 mL in Ethylene Diamine Tetraacetic Acid [EDTA] tube, and 2 mL in plain tube) were withdrawn from each participant for complete blood count and quantitative determination of CRP, respectively.
Demographic and clinical data of the participants were obtained from the electronic hospital system that is specified for recording patient's data.
Extraction of SARS-CoV-2 RNA
Full automated purification of COVID-19 RNA was done on QIAcube device using the QIAamp RNA viral extraction kit (Qiagen, Heiden, Germany) following the manufacturer's instructions.
Real-Time Reverse Transcription Quantitative-PCR for SARS-CoV-2 RNA
Detection and quantitation of SARS-CoV-2 were performed by Genesig RT-PCR assay (Primerdesign Ltd, School Lane, Chandler's Ford, UK) that is TaqMan based and targets the RNA dependent RNA polymerase (RdRp) gene. Reverse transcription and target amplification were done in one step approach on DTlite 4 RT-PCR system (DNA-Technology, Varshavskoe Sh 125zh, Moscow, Russia) according to instructions provided by the manufacturer.
Viral load of COVID-19 virus was determined by generating a standard curve using positive control template of SARS-CoV-2 provided with the kit and following manufacturer's instructions published on January 28, 2021 in Genesig Standard kit handbook. In brief, five 10-fold serial dilutions were prepared from a positive control template of 2 ×105 copies/µL. After target amplification by RT-PCR, the log copy number of viral genome was plotted against its respective cycle threshold (Ct) value. Interpretation of sample results was done following manufacturer' recommendations and their viral loads were calculated from the previously performed standard curve according to the following equation:
log10 SARS-CoV-2 RNA copies/µL= −0.3333*Ct + 10.6.
Rapid Antigen Detection Test of SARS-CoV-2
Rapid detection of SARS-Cov-2 antigen was performed by using BIOCREDIT/COVID-19 antigen kit (RapiGEN Inc., Gyeonggi-do, Korea; henceforth Rapigen) that is a qualitative assay based on lateral flow immunochromatographic technique. The test device has two lines: test line and control line. At the test line, there is SARS-CoV-2 antigen-specific monoclonal antibodies that are conjugated to nanoparticles of colloidal gold. If the tested sample contains SARS-CoV-2 antigen, a complex of antigen–antibody gold conjugate will be formed and appears as a band of black color on the test line.
The rapid antigen assay was performed in BSL-2 laboratory by well-trained personnel who did not know the results of RT-PCR. The steps recommended by the manufacturer were followed. Careful mixing of NP swabs in the assay diluent tube was done by swirling the swab 5 to 10 times with pressing its head against sides of the tube. Then four drops were squeezed from the prepared assay diluent tube into the sample site of the device. The results were read within 5 to 8 minutes separately by two trained personnel conferring with a third one if there was a disagreement in the result reading. The result was considered; positive if there were two visible bands (red control line and black test line), negative if there was only one red band at the control line, and invalid if no band appeared at the control line.
Complete Blood Count
Complete blood count with neutrophil and lymphocyte percentage was performed on automated hematology analyzer (Beckman, Brea, California, USA). Then NLR was calculated as follows; absolute neutrophil count/absolute lymphocyte count × 100.
C-Reactive Protein Assay
Quantitation of CRP was performed on COBAS C311 system (Roche, Basel, Switzerland) based on immunoturbidimetric assay. CRP level was interpreted as normal if less than 6 mg/L.
Statistical Analysis
Data were analyzed using SPSS 22 (IBM Corp., Armonk, New York, United States). Continuous variables were presented as median (range), while qualitative variables were described as frequency (%). Categorical variables were compared by chi-squared test and p-value less than 0.05 was considered statistically significant. Receiver operating characteristics (ROC) curves were used to determine the cutoff levels for CRP and NLR with calculation of the corresponding sensitivity, specificity, and accuracy.
Results
During the study period, 1877 NP swabs from SARS-CoV-2 suspected subjects were received in the COVID-19 RT-PCR laboratory. Out of these suspected subjects, 530 (28.2%) were COVID-19 positive by RT-PCR. The ratio of positive to negative RT-PCR results was 0.39:1 (►Fig. 1).
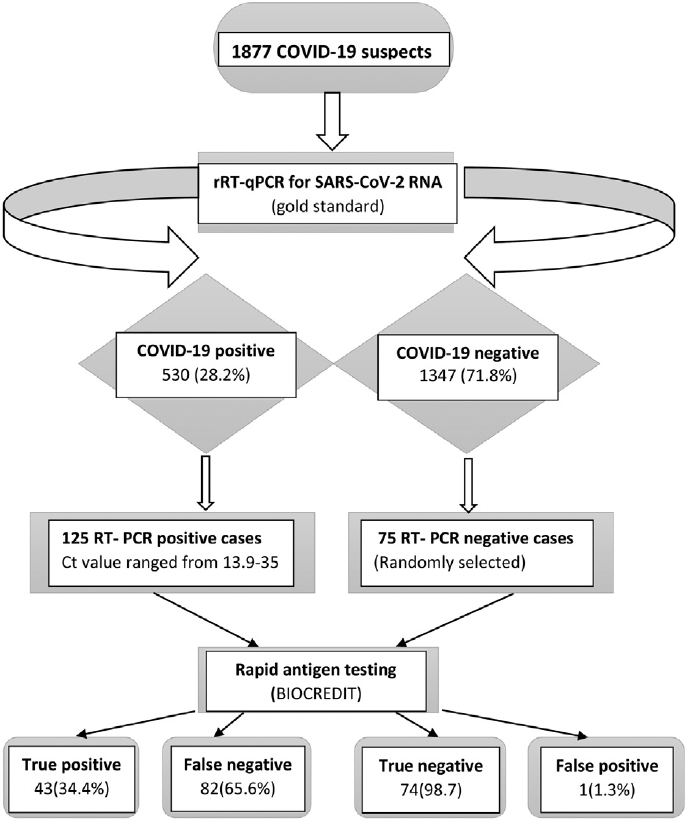
- Flowchart indicating sample processing and results. COVID-19, coronavirus disease 2019; rRT-PCR Ct, real-time reverse-transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Due to unavailability of the evaluated RAD kit (throughout the whole study period), only 200 PCR-characterized COVID-19 suspect were included in this study. Of these 200 participants, 75 (37.5%) were RT-PCR negative, while 125 (62.5%) were RT-PCR positive with a median PCR-Ct value of 25.5 (range: 13.9–35) equivalent to median (range) viral load of 5 ×102 (3.70 × 106–0.34) copies/µL.
Demographic, Clinical, and Laboratories Characteristics
The median age of the participants was 59 years (range: from 23 to 96 years) with male/female ratio of 1.35:1 (115 males and 85 females). Among the participants, 5 (2.5%) were of no clinical symptoms suggesting infection with COVID-19, but they had a positive history of contact to PCR-confirmed COVID-19 patient. Of these asymptomatic subjects, three participants (75%) were PCR-negative and two participants (25%) were PCR positive for SARS-CoV-2 RNA. The viral load of these two asymptomatic PCR-positive subjects was 23.318 and 2.936 copies/µL.
Out of 195 symptomatic participants, 123 (63.1%) were PCR-positive and 72 (36.9%) were PCR-negative. Respiratory symptoms (as cough, sore throat, and dyspnea) were the most frequent symptoms in both PCR-positive and negative subjects (53.6 and 44%, respectively). Gastrointestinal (GI) symptoms (as vomiting, diarrhea, and loss of taste) were the only presenting symptoms in 3.2% of PCR-positive and in 14.7% of PCR-negative patients. Those presented with both respiratory and GI symptoms were 66(33%) of the studied participants (42/125; 33.6% of PCR-positive and 24/75; 32% of PCR-negative). According to oxygen saturation and respiratory rate, the 125 PCR-confirmed participants were classified into 73 (58.4%) severe COVID-19 patients (O2 saturation ≤ 92% and respiratory rate > 30/min) and 52 (41.6%) nonsevere COVID-19 patients.
CRP level was significantly elevated among PCR-confirmed COVID-19 patients than PCR-negative patients with median (range) values of 45 (2.4–305) and 22 (1–250) mg/L, respectively (p-value < 0.001). Similarly, significant higher median value of NLR (6.3) was detected in PCR-confirmed than PCR-negative patients (2.6) (p-value < 0.001) (►Table 1).
| SARS-CoV-2 suspected subjects no (%) | p-Value | |||
|---|---|---|---|---|
| All subjects | RT-PCR positive | RT-PCR negative | ||
| Number | 200 (100%) | 125 (62.5%) | 75 (37.5%) | – |
| Age | 0.9 | |||
| Mean ± SD | 57.8 ± 15.6 | 57.8 ± 14.8 | 57.9 ± 15.9 | |
| Median (min–max) | 59 (23–96) | 60 (23–90) | 57 (25–96) | |
| Sex | 0.08 | |||
| Male | 115 (57.5) | 66 (52.8) | 49 (65.3) | |
| Female | 85 (42.5) | 59 (47.2) | 26 (34.7) | |
| Clinical pictures | 0.29 | |||
| Asymptomatic | 5 (2.5) | 2 (1.6) | 3 (4) | |
| Symptomatic | 195 (97.5) | 123 (98.4) | 72 (96) | |
| Fever alone | 14 (7) | 10 (8) | 4 (5.3) | 0.47 |
| Respiratory symptoms alone | 100 (50) | 67 (53.6) | 33 (44) | 0.18 |
| GI symptoms alone | 15 (7.5) | 4 (3.2) | 11 (14.7) | 0.003 |
| Combined respiratory and GI | 66 (33) | 42 (33.6) | 24 (32) | 0.81 |
| Clinical severity | ||||
| Severea | 106 (53) | 73 (58.4) | 33 (44) | 0.04 |
| Nonsevere | 94 (47) | 52 (41.6) | 42 (56) | |
| CRP (mg/L) | < 0.001 | |||
| Median (min–max) | 37.7 (1–305) | 45 (2.4–305) | 22 (1–250) | |
| TLC (x103/µL) | < 0.001 | |||
| Median (min–max) | 8.8 (1.2–21.3) | 10 (3–21.3) | 7.7 (1.2–20.5) | |
| ANC (x103/µL) | < 0.001 | |||
| Median (min–max) | 6.75 (0.4–16–8) | 8.2 (2–16.8) | 4.6 (0.4–16.8) | |
| ALC (x103/µL) | < 0.001 | |||
| Median (min–max) | 1.4 (0.4–4.5) | 1.2 (0.4–4.5) | 1.7 (0.6–2.9) | |
| NLR | < 0.001 | |||
| Median (min–max) | 5 (0.67–17.86) | 6.3 (1–17.86) | 2.6 (0.67–8.9) | |
Abbreviations: ALC, absolute lymphocyte count; ANC, absolute neutrophil count; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; GI, gastrointestinal; max, maximum; min, minimum; NLR, neutrophil to lymphocyte ratio; SD, standard deviation; TLC, total leucocyte count.
a Severe COVID19: if oxygen saturation ≤ 92% and respiratory rate > 30/min.[10]
ROC curve analysis showed that the best cutoff value of CRP for predicting COVID-19 was 31.6 mg/L with a sensitivity of 72%, a specificity of 60%, and test accuracy of 67.5%. Also, ROC curve analysis determined the value of 3.57 as a cutoff value of NLR for diagnosis of COVID-19 with a sensitivity, specificity, and accuracy of 84, 68, and 78%, respectively (►Fig. 2).
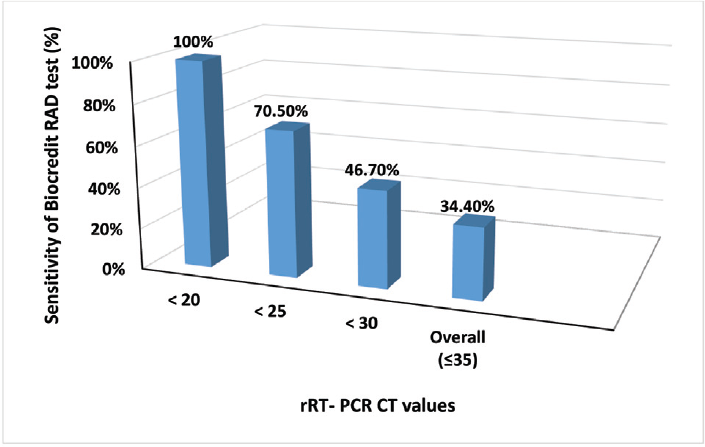
- Sensitivity of BIOCREDIT coronavirus disease 2019 (COVID-19) antigen test in nasopharyngeal specimens with different real-time reverse-transcription polymerase chain reaction cycle threshold (rRT-PCR Ct) values. RAD, rapid antigen detection.
Overall Diagnostic Performance of BIOCREDIT/COVID-19 Antigen Test
Compared with RT-PCR results, the evaluated BIOCREDIT/COVID-19 antigen test was able to identify 43 (34.4%) of 125 RT-PCR positive specimens as a true positive and 74 (98.7%) of 75 RT-PCR negative specimens as a true negative. PCR-Ct median value of specimens that identified by BIOCREDIT as a true positive was 18.9, equivalent to median viral load of 7.96 × 104 copies/µL. Based on the analysis of Ct values of RT-PCR, a Ct value of 22.7 (equivalent to 4.31 × 103 copies/µL) was the cutoff value for the best discrimination between COVID-19 positive and negative samples with this kit and hence assumed as a detection limit of the evaluated RAD test.
Out of 125 PCR positive specimens, 82 (65.6%) were interpreted as false negative by BIOCREDIT/antigen test and were of PCR-Ct median (range) values of 28.75 (20.8–35), corresponding to viral load median (range) of 41.49 (1.85 × 104–0.34) copies/µL. Interestingly, only one specimen (1.3%) was false positive by BIOCREDIT/antigen test.
Performance of BIOCREDIT/COVID-19 Antigen Test in Different Subgroups
Regarding the impact of patient clinical presentation, sensitivity of BIOCREDIT test for detection of COVID-19 was increased from 0% among asymptomatic participants to 35% among symptomatic patients. Similarly, its sensitivity was increased to 40% in patients presented only with fever and decreased to 25% in those presented only with GI symptoms. Moreover, its sensitivity was significantly increased from 3.8% in nonsevere to 56.2% in severe COVID-19 patients (►Table 2).
| Subgroups | Number | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|
| All participants | 200 | 34.4% | 98.7% | 58.5% |
| According to patients' symptoms | ||||
| Asymptomatic | 5 | 0 | 100% | 60% |
| Fever alone | 14 | 40% | 100% | 57.1% |
| Respiratory symptoms alone | 100 | 34.3% | 97% | 55% |
| GI symptoms alone | 15 | 25% | 100.0% | 80% |
| Both respiratory and GI symptoms | 66 | 35.7% | 100.0% | 59.1% |
| According to severity | ||||
| Severe | 106 | 56.2% | 97% | 68.9% |
| Nonsevere | 94 | 3.8% | 100% | 46.8% |
| According to viral loada | ||||
| High viral load | 16 | 100% | – | 100% |
| Low viral load | 184 | 24.8% | 98.7% | 54.9% |
| ≥ 1000 copies /µLb | 57 | 75.4% | – | 75.4% |
| < 1000 copies /µL | 143 | 0 | 98.7% | 51.7% |
| According to CRP level | ||||
| ≤ 31.6 mg/Lc | 80 | 17.1% | 100.0% | 63.75% |
| >31.6 mg/L | 120 | 41.1% | 96.75 | 55% |
| According to NLR | ||||
| ≤ 3.57[d] | 71 | 25% | 100% | 78.9% |
| > 3.57 | 129 | 36.2% | 95.8% | 47.3% |
Abbreviations: COVID-19, coronavirus disease 2019; CRP, C-reactive protein; GI, gastrointestinal; NLR, neutrophil to lymphocyte ratio; PCR-Ct, polymerase chain reaction cycle threshold; ROC, receiver operating characteristics; WHO, World Health Organization.
a High viral load: if PCR-Ct value ≤ 18 (equivalent to viral copies/µL > 158865.647); low viral load: if PCR negative or PCR positive with Ct value > 18 (equivalent to viral copies/µL ≤ 158865.647).
b Contagious patients as assumed by the WHO.[9]
c Cutoff value determined by ROC curve at which CRP could be used as a predictor of COVID-19 infection.
d Cutoff value determined by ROC curve at which NLR could be used as a predictor of COVID-19 infection.
According to viral load, the BIOCREDIT/antigen test showed an increasing sensitivity with the increase in the viral load of the specimens. For specimens with PCR-Ct value less than 20 (n = 26), less than 25 (n = 61), less than 30 (n = 92), less than or equal to 35 (n = 125) with viral loads of greater than 3.42 × 104, 7.4 × 102, 15.9, and 0.34 copies/µL, respectively, its sensitivity of detecting COVID-19 was 100, 70.5, 46.7, and 34.4%, respectively (►Fig. 2). Among contagious patients (those with viral load of 1 × 103 copies/µL or higher as assumed by the WHO),[9] BIOCREDIT test detected 43 of 57 PCR-positive specimens with a sensitivity of 75.4%. Moreover, its sensitivity was increased from 24.8% among 184 samples with a low viral load (Ct > 18, ≤ 1.59 × 104 copies/µL) to 100% among 16 samples with a high viral load (Ct ≤ 18, > 1.59 × 104 copies/µL) (►Table 2).
According to CRP level, considering CRP level of 31.6 mg/L as a cutoff value for prediction of COVID-19 infection, sensitivity of the BIOCREDIT test was increased from 17.1% among participants with CRP level less than or equal to 31.6 mg/L to 41.1% among those with CRP level greater than 31.6 mg/L.
Regarding NLR, taking NLR of 3.57 as a cutoff value for prediction of COVID-19 infection, sensitivity of the BIOCREDIT test was increased from 25% among participants with NLR less than or equal to 3.57 to 36.2% among those with NLR greater than 3.57 (►Table 2).
Performance of the BIOCREDIT/Antigen Test in Combination with NLR or CRP
When BIOCREDIT/antigen test was combined with NLR, its sensitivity and accuracy for detecting COVID-19 were increased from 34.4 to 89.4% (for sensitivity) and from 58.5 to 80% (for accuracy). However, its specificity was decreased from 98.7 to 67%. Similarly, BIOCREDIT/antigen test showed an increase in its sensitivity (81.6%) and accuracy (70.5%) and decrease in its specificity (59%) when combined with CRP determination (►Fig. 3).

- Performance of BIOCREDIT coronavirus disease 2019 antigen test alone and in combination with C-reactive protein (CRP) or neutrophil/lymphocyte ratio (NLR).
Discussion
BIOCREDIT/COVID-19 antigen test is one of the common commercially available rapid tests for SARS-CoV-2 antigen in Egypt. The intended use of this test is rapid testing of COVID-19 suspect in triage stations to help control the disease spread by rapid identification and hence immediate isolation of COVID-19 cases. Therefore, the present study evaluated the performance of this RAD test as a single diagnostic test and when combined with CRP level or NLR ratio in a group of suspected COVID-19 subjects with different clinical and laboratory presentations.
In the current study, as compared with rRT-PCR results (the gold standard laboratory method for detection of SRAS-CoV-2 infection), BIOCREDIT/COVID-19 test identified truly all PCR-negative samples (with exception of only one specimen identified as false positive) yielding a specificity of 98.7% that is in line with that reported by the manufacturer (98%). Thick and highly viscous mucous specimens could be the cause of false positive results when tested with the antigen detection kit.[11]
Our data showed that BIOCREDIT/COVID-19 test had a sensitivity of 34.4% and detected truly all COVID-19 positive specimens with viral load of greater than or equal to 4.31 × 103 copies/µL (equivalent to PCR-Ct value of less than or equal to 22.7). Although this detected sensitivity (34.4%) was lower than sensitivity reported by the manufacturer (92%), it was in line with other previous independent studies that reported a sensitivity from 11.1 to 45.7% for BIOCREDIT/COVID-19 test.[12,13] Also, two earlier studies performed at other two different governorates in Egypt for the evaluation of the same kit recorded sensitivities of 52.5% and 43.1% that are still lower as compared with the manufacturer (92%) but higher as compared with our study.[14,15]
Generally, the discrepancy in test sensitivity across different studies could be attributed to differences in many factors such as the presenting symptoms, duration from symptoms onset to sample collection, type of the investigated specimen, and processing of specimens. The reason of our remarkable low overall sensitivity of the BIOCREDIT test as compared with other studies could be the inclusion of high percentage (64.8%) of COVID-19 positive specimens with viral load less than 4.31 × 103 copies/µL (the assumed detection limit of this evaluated kit from our findings).
In this study, we hypothesized that the performance of RAD could differ according to the clinical and laboratory presentation of patients. Therefore, we tried for the first time to perform a subgroup analysis for investigating the differential effect of these factors on the sensitivity of the BIOCREDIT/COVID-19 test to take the maximum benefit from its use during COVID-19 pandemic.
In accordance with the clinical presentation of the patients, we found that sensitivity of BIOCREDIT/COVID-19 test is better among symptomatic than among asymptomatic patients (35 and 0%, respectively). This could be due to higher replication of SARS-CoV-2 in the pharynx of symptomatic patients than asymptomatic.
Based on the type of the presenting symptoms, sensitivity of BIOCREDIT test was highest among those presented with fever alone (40%) and was better among those presented with either respiratory symptoms alone or with both respiratory and GI symptoms (34.3 and 35.7%, respectively) than those presented only with GI symptoms (25%). Similarly, Berger et al observed that Standard Q test and Panbio test (rapid tests for SAR-Cov-2 antigen from other manufacturers) had the highest sensitivity (93.8%) among patients presented with fever and cough, and the lowest sensitivity (73.8%) among patients presented with nonspecific signs.[16]
Although sensitivities of different rapid tests for detection of SARS-CoV-2 antigen are widely variable across studies, it is noted that these rapid tests usually perform more better in specimens with higher viral load.[17] Likewise, our study found that the sensitivity of the BIOCREDIT/antigen test was highest in specimens with high viral load. Its sensitivity was 100% for 26 specimens with SARS-CoV-2 greater than 3.42 × 104 copies/µL, decreased to 70.5% for 61 specimens with SARS-CoV-2 greater than 7.4 × 102 copies/µL, dropped more to 46.7% for 92 specimens with SARS-CoV-2 greater than 15.9 copies/µL. The lowest sensitivity (34.5%) was for 125 specimens with SARS-CoV-2 greater than 0.34 copies/µL.
The overall sensitivity of BIOCREDIT/COVID-19 test was significantly higher (100%) among specimens with higher viral load than its sensitivity (24.8%) among specimens with low viral load. This finding agrees with Khairat et al who reported a substantial increase in the sensitivity of BIOCREDIT/COVID-19 test with the increase in the viral load; they reported a sensitivity of 45% for specimens with low viral load (Ct values > 18.57) and 60% for specimens with high viral load (Ct values < 18.57).[14] Also, Abdelrazik et al recorded a higher sensitivity of BIOCREDIT test (93.4%) in specimens with high viral load (Ct values < 25.5).[15]
The WHO assumed 1 × 106 SARS-CoV-2 copies/mL as a cutoff level of contagiousness.[9] In the present study, the sensitivity of the evaluated BIOCREDIT/antigen test, for this viral load compatible with contagiousness, was 74.5% that is lower than Panbio (Abbott, United States) and Standard Q (Roche, Switzerland) COVID-19 RAD tests whose sensitivities for detection of the contagious COVID-19 patients were reached to 95.7%.[16]
Up to our knowledge, none of the previous studies had evaluated the performance of any of the available COVID-19 rapid antigen tests on the base of the patient's routine laboratory results. This study demonstrated that the sensitivity of the BIOCREDIT/antigen test to identify COVID-19 cases could be raised from 34.4% (if applied among any suspect regardless of his routine laboratory results) to 41.1% or 36.2% if its use as a diagnostic tool for COVID-19 was confined only to those presented with CRP level greater than 31.6 mg/L or to those presented with NLR greater than 3.57, respectively. Therefore, we suggest that NLR and CRP level could play an important role within COVID-19 diagnostic strategies that use rapid SARS-CoV-2 antigen detection tests.
Sensitivities of either CRP (72%) or NLR (84%) for prediction of COVID-19 were higher than sensitivity demonstrated by BIOCREDIT/COVID-19 antigen test (34.4%). However, the specificities of these routine laboratory tests (60% for CRP, 68%, for NLR) were lower than specificity of BIOCREDIT/COVID-19 antigen (98.7%). Therefore, to improve the shortcomings of each test alone, the present study did a combination assay for diagnosis of COVID-19 cases. Combined use of BIOCREDIT/COVID-19 test with CRP showed an improvement in the sensitivity for detection of COVID-19 patients to 81.6%, but the specificity decreased to 59%. Also, when we combined BIOCREDIT/COVID-19 test with NLR, the sensitivity increased higher to 89.4% but the specificity decreased to 67%.
Therefore, our findings suggest that combined assay of BIOCREDIT/COVID-19 antigen test with NLR could rapidly identify high percentage of true positive COVID-19 patients who urgently need appropriate management and isolation measures to limit SARS-CoV-2 spread in the community. Also, combination assay will decrease the percentage of false negative results and hence reduce the need for confirmation by rRT-PCR tests to overcome overwhelmed diagnostic laboratories and global PCR-reagent shortages.[18]
Conclusions
BIOCREDIT/COVID-19 antigen test should not be used as a single test for diagnosis of COVID-19 infection particularly in asymptomatic subjects. However, due to its fast results (within minutes), simple use, and no need for special expensive equipment, this kit could play a significant role in the diagnosis of COVID-19 if only used in combination with either NLR or CRP level in subjects with certain clinical criteria. Future studies including large sample size of asymptomatic subjects are recommended for further evaluation of this kit.
Ethical Approval
Ethical Committee, Faculty of Medicine–Mansoura University (MFM-Institutional Research Board), Egypt approved this study with a code number of R.21.09.1441. A written informed consent was obtained from all study participants before sample collection. All samples were analyzed anonymously.
Acknowledgments
We would like to thank all teamwork of COVID-19 PCR-laboratory and quarantine department in the hospital for their valuable efforts.
Conflicts of Interest
There was no conflict of interest between the authors. Also, there was no conflict of interest with either the manufacturer or the supplier of the evaluated kit, the kit was procured on the expense of the authors, and there was no influence of the kit supplier on the results presented in this study.
References
- Three emerging coronaviruses in two decades. Am J Clin Pathol. 2020;153(04):420-421.
- [CrossRef] [PubMed] [Google Scholar]
- China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China,N Engl J Med. 20192020;382(08):727-733.
- [Google Scholar]
- Director-General's opening remarks at the media briefing on COVID-19-March, 2020. Accessed on March 27, 2022, at: https://www.who.int/directorgeneral/speeches/detail/who-director-general-s-opening-remarks-at-the-media briefing-on-covid-19 11-march-2020
- [Google Scholar]
- Laboratory diagnosis of COVID-19. J Pediatr (Rio J). 2021;97(01):7-12.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of the neutrophil-to-lymphocyte ratio and C-reactive protein level for coronavirus disease 2019 (COVID-19) Scand J Clin Lab Invest. 2020;80(07):536-540.
- [CrossRef] [PubMed] [Google Scholar]
- Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20(05):453-454.
- [CrossRef] [PubMed] [Google Scholar]
- SARS-CoV-2 Diagnostic Pipeline. 2020 Accessed on March 27, 2022, at: https://www.finddx.org/covid-19/pipeline/
- [Google Scholar]
- Group TEC-19 microbiological laboratories. Meta-analysis of the clinical performance of commercial SARS-CoV-2 nucleic acid, antigen and antibody tests up to 22 August 2020. MedRxiv. 2020;18 2020
- [Google Scholar]
- COVID-19 target product profiles for priority diagnostics to support response to the COVID-19 pandemic, v.1.0. September 28. 20202020 Accessed on March 27, 2022, at: https://www.who.int/publications/m/item/covid-19-target-product-profiles-for-priority-diagnostics-to-support-response-to-the-covid-19-pandemic-v.0.1.
- [Google Scholar]
- Management protocol for COVID-19 patients. COVID-19 Ministry of Health and Population, Egypt. version 1.5. September 2021. Accessed on March 27, 2022, at: https://www.researchgate.net/publication/354694237_Management_Protocol_for_COVID-19_Patients_COVID19_Ministry_of_Health_and_Population_Egypt_version_15_September_2021/stats#fullTextFileContent
- [Google Scholar]
- Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol J. 2020;17(01):177.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of a rapid diagnostic assay for detection of SARS-CoV-2 antigen in nasopharyngeal swabs. J Clin Microbiol. 2020;58(08):e00977-e20.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J Clin Virol. 2020;129 104500
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of two rapid antigen tests for detection of SARS-CoV-2 virus. Int J Microbiol Biotechnol. 2020;5(03):131-134.
- [CrossRef] [Google Scholar]
- Potential use of antigen-based rapid test for SARS-CoV-2 in respiratory specimens in low-resource settings in Egypt for symptomatic patients and high-risk contacts. Lab Med. 2021;52(02):e46-e49.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of two commercial SARS-CoV-2 antigen-detecting rapid tests at the point of care in community-based testing centers. PLoS One. 2021;16(03):e0248921.
- [CrossRef] [PubMed] [Google Scholar]
- Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays interim guidance. 11 September. Accessed on March 27, 2022, at: https://apps.who.int/iris/bitstream/handle/10665/334253/WHO-2019-nCoV-Antigen_Detection-2020.1-eng.pdf.
- [Google Scholar]
- Rethinking Covid-19 test sensitivity - a strategy for containment. N Engl J Med. 2020;383(22):e120.
- [CrossRef] [PubMed] [Google Scholar]





