Translate this page into:
Evaluation of renal function in subclinical hypothyroidism
Address for correspondence: Dr. Vijayetha P. Patil, Department of Biochemistry, SDM College of Medical Sciences and Hospital, Sattur, Dharwad, Karnataka, India. E-mail: drvijayetha@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
INTRODUCTION:
Patients with subclinical hypothyroidism (SCH) have a few or no symptoms or signs of thyroid dysfunction and thus by its very nature, SCH is a laboratory diagnosis. Serum creatinine is elevated and glomerular filtration rate (GFR) values are reversibly reduced in overt hypothyroid patients. We hypothesize that SCH also may be associated with low GFR.
AIMS AND OBJECTIVES:
The objective of this study was (1) to know the effect of SCH on kidney function, (2) to find the correlation between the renal function parameter creatinine, estimated GFR (eGFR), and thyroid-stimulating hormone (TSH), and (3) to know if creatinine values can be predicted by TSH values in SCH cases.
MATERIALS AND METHODS:
This is a hospital-based cross-sectional study for 1 year. A total of 608 subjects of either sex were included in the study and were divided into 3 groups: (1) SCH, (2) overt hypothyroidism (OHT), and (3) euthyroidism (ET). TSH, free triiodothyronine, free thyroxine, and serum creatinine were estimated and eGFR was calculated using modification of diet in renal disease study equation and the chronic kidney disease epidemiology collaboration equations.
RESULTS:
Serum creatinine levels were higher and eGFR was lower significantly in the subclinical hypothyroid group when compared to the control ET group (P < 0.001). The overtly hypothyroid group had significantly higher levels of serum creatinine and lower eGFR when compared to both the groups (P < 0.001). Significant correlation between TSH, creatinine, and eGFR was found in OHT group only. Linear regression analysis showed the regression in creatinine upon TSH is attributable to 44.5% among OHT group, 48.2% in SCH group.
CONCLUSION:
It can be concluded that the SCH group behaves biochemically similar to OHT group and changes in serum creatinine reflect tissue hypothyroidism in SCH cases.
Keywords
Creatinine
estimated glomerular filtration rate
renal dysfunction
subclinical hypothyroidism
thyroid-stimulating hormone
tissue hypothyroidism
Introduction
Subclinical hypothyroidism (SCH) is defined as an elevated serum thyroid-stimulating hormone (TSH) above the defined upper limit of the reference range, with a serum free thyroxine (FT4) within the reference range. SCH cases present with few or no symptoms or signs of thyroid dysfunction and thus by its very nature SCH is a laboratory diagnosis. The prevalence of SCH in the United States adult population is 4%–8.5%.[1] Various epidemiological studies in India shows a prevalence rate of SCH varying between 9% and 11.4%.[2] The progression to overt hypothyroidism (OHT) is approximately 2%–5%/year. Due to its asymptomatic nature, the SCH cases are not detected clinically[2] and also its relation to the kidney function is not well established. There is paucity of data in Indian population. Patients with SCH report more symptoms than ET individuals, but fewer symptoms than overtly hypothyroid participants.[1] There is controversy in management of patients with a serum TSH level <10 μIU/L. There is inadequate literature in this area and statements may often be an expert panel opinion rather than strictly evidence based.
There is a well-known interaction between thyroid and kidney functions. Thyroid hormones are involved in the growth, development, and physiology of the kidney.[3] Hypothyroid state is associated with significant derangement in biochemical parameters of renal function.[45] Serum creatinine is elevated and glomerular filtration rate (GFR) values are reversibly reduced in overt hypothyroid patients than in ET subjects.[36789] We hypothesize that SCH also may be associated with low GFR. Renal dysfunction can be actually emphasized as the reflection of tissue hypothyroidism. There is insufficient or no evidence to support an association between SCH and its systemic effects.[1] The basis for treatment in SCH is not clear yet. This investigation would help us to find out whether SCH manifests on the organ function, i.e., renal function. If so, it would help in planning the treatment for SCH.
Hence, this study was taken up to evaluate the renal function by estimating serum creatinine and estimated GFR (eGFR) in SCH patients with the following objectives:
-
To know the effect of SCH on kidney function
-
To find the correlation between the renal function parameter creatinine, eGFR, and TSH
-
To know if creatinine values can be predicted by TSH values in SCH cases.
Materials and Methods
It is a retrospective cross-sectional study. All the cases detected with SCH for 1 year were taken for the study. The data were collected from patients coming to SDM hospital for consultation in outpatient department (OPD) and from medical records. From all the patients who had undergone blood tests for creatinine and thyroid function testing between January 2015 and January2016, one set of results was collected.
Inclusion criteria
The study includes all the subjects in the age group of 18–70 years of either sex attending the OPD in SDM hospital, Dharwad. The subjects were divided into three groups after laboratory investigations:
-
Group 1: SCH: Subjects having higher TSH and free triiodothyronine (FT3), FT4 within the normal reference range
-
Group 2: Overt hypothyroid (OHT): Subjects having higher TSH and low FT3 or FT4 and
-
Group 3: Euthyroid (ET): Subjects having TSH, FT3, and FT4 all within the normal reference range.
Exclusion criteria
Subjects who are known cases of thyroid disorders, on treatment with drugs such as amiodarone, lithium, iodine, antithyroid drugs, patients having renal disorders, liver disorders, diabetes, hypertension, and other chronic inflammatory disorders were excluded from the study.
A uniform protocol was followed for sample collection and analysis of the tests in the laboratory. 5 mL of blood sample was drawn under aseptic precautions. After 30 min, it was centrifuged and serum separated was sorted in 2 aliquots; one for thyroid profile and the other for estimation of creatinine. The tests were done immediately on the same day.
Methods of estimation
TSH, FT4, and FT3 were estimated by chemiluminescence immunoassay technology in fully automated immunoassay analyzer from Siemens Advia Centaur CP. The reference range followed in the laboratory for TSH is 0.35–5.5 μIU/L, FT3 is 2.3–4.2 pg/ml, and FT4 is 0.89–1.76 ng/dl.
Creatinine was estimated by modified kinetic Jaffe's method in fully automated Siemens Dimension RXL max chemistry analyzer. Calibrator for creatinine is traceable to isotope-dilution mass spectrometry (IDMS) primary reference measurement procedure. All analyses were performed in accordance with manufacturer's instructions. The reference range followed in the laboratory for creatinine is 0.5–1.3 mg/dl.
The modification of diet in renal disease (MDRD) study equation and the chronic kidney disease epidemiology collaboration (CKD-EPI) equation are the most widely used IDMS traceable equations for estimating GFR in patients aged 18 and over. We have used both the equations for eGFR calculation.[1011]
The following is the IDMS-traceable MDRD study equation (for creatinine methods calibrated to an IDMS reference method):
GFR (mL/min/1.73 m2) =175× (Scr)−1.154× (Age)−0.203× (0.742 if female) × (1.212 if African American) [Table 1].
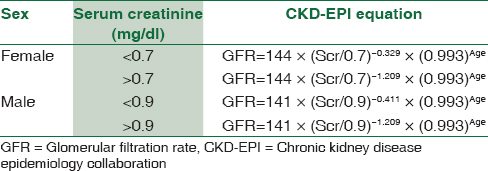
The chronic kidney disease epidemiology collaboration (CKD-EPI)equation for estimating glomerular filtration rate is given in Table 1.
Statistical analysis
All the findings are expressed as mean ± standard deviation. The P < 0.05 was considered statistically significant, P < 0.01 as highly significant, and P < 0.001 as very highly significant. Analysis was performed by various statistical tests such as general linear model (GLM), ANOVA, Pearson's correlation, and linear regression using IBM® SPSS® statistics version 20.0. (IBM Corporation, New York, USA). Licensed officially to SDM College of Medical Sciences, Dharwad - 580 009, India, by IBM-SPSS Corporation, Bengaluru.
Ethical clearance has been obtained from the Institutional Ethical Clearance Committee, SDM College of Medical Sciences and Hospital, Dharwad. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the Declaration of Helsinki 1964 and its later amendments or comparable ethical standards.
Results
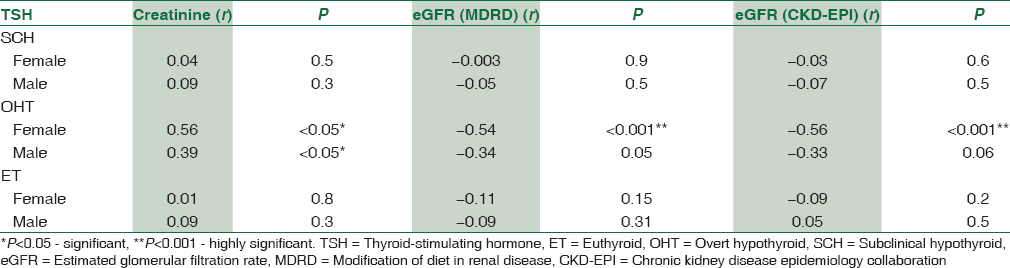
A total of 608 subjects were included in the study. Table 2 shows the results of one-way ANOVA for comparison of means of investigation reports between overall groups under study. We found a highly significant difference in the mean values of FT3, FT4, TSH, serum creatinine, eGFR by MDRD, and eGFR by CKD-EPI equation among all the groups as shown in Table 2. Similar findings were also seen in post hoc test. Table 3 shows a linear trend of increase in creatinine values from ET controls to SCH to OHT groups.
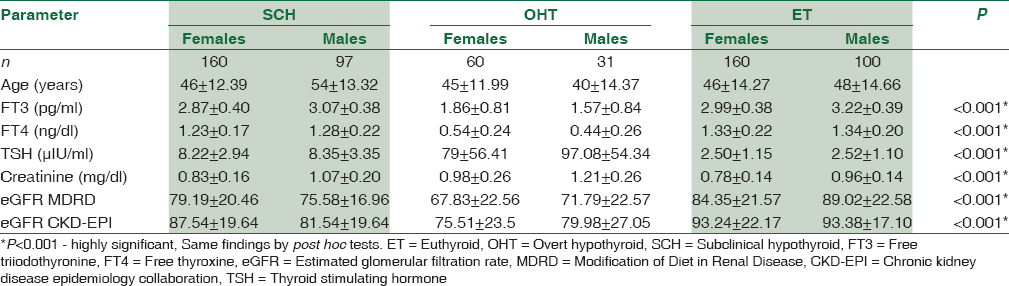

Pearson's correlation studies reveal that TSH levels were well correlated with serum creatinine (r = 0.54, P < 0.001) in the OHT group only. No correlation was found with ET and SCH groups (r = 0.034, r = 0.06, respectively). Similarly, a significant negative correlation between TSH and eGFR was found only in OHT group. The results are summarized in Table 4.
We found a very strong positive correlation between eGFR calculated by MDRD and eGFR by CKD-EPI equations in all the subjects (r = 0.96, P < 0.001) as shown in Figure 1. Correlation of the same in different groups is shown in Table 5.
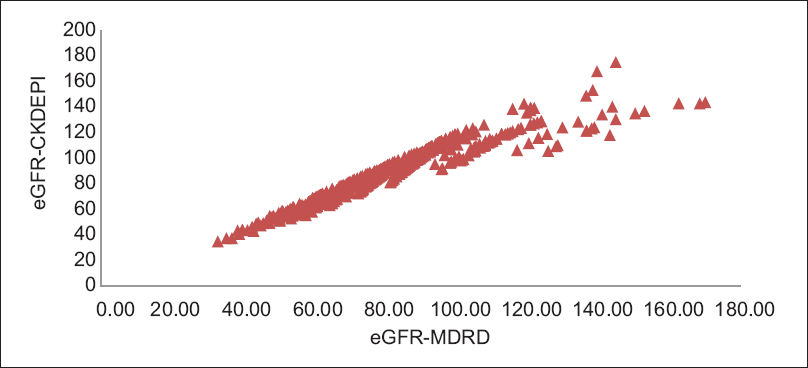
- Correlation between estimated glomerular filtration rate - modification of diet in renal disease and estimated glomerular filtration rate - chronic kidney disease epidemiology collaboration in all three groups. R = 0.96, P < 0.001 highly significant

Linear regression analysis in GLM model [Table 6] shows that the linear regression in creatinine based on the TSH values is attributable to the extent of 44.5% among the OHT group (adjusted R2 = 0.445 with residual error of 0.043). However, the model fits best in the SCH group where 48.2% can be attributed (adjusted R2 = 0.482 with residual error 0.024) indicates a regression model comparable to the OHT group if not better. This compares better in the OHT group than in the control ET group where adjusted R2 = 0.23 only. The residual error of 0.024 which is half of the error in OHT group, 0.043 also indicates a better model fit.
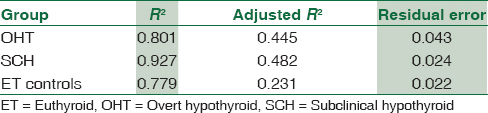
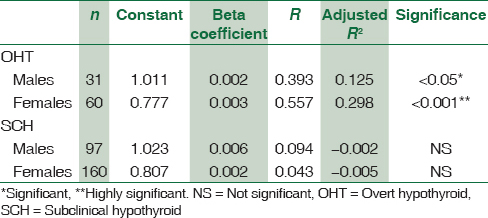
The regression coefficients have been shown in Table 7 in respect of OHT and SCH groups only since the control ET group has very low adjusted R2. Gender offers a significant covariate contribution in the model of OHT group. Hence, its effect has been eliminated by calculating the regression coefficient separately for the genders. Similarly, the same gender-wise segregation has been done in the SCH group for calculating the regression coefficient to fit into the model. The same is proved in the residual P-P plots shown in Figure 2.
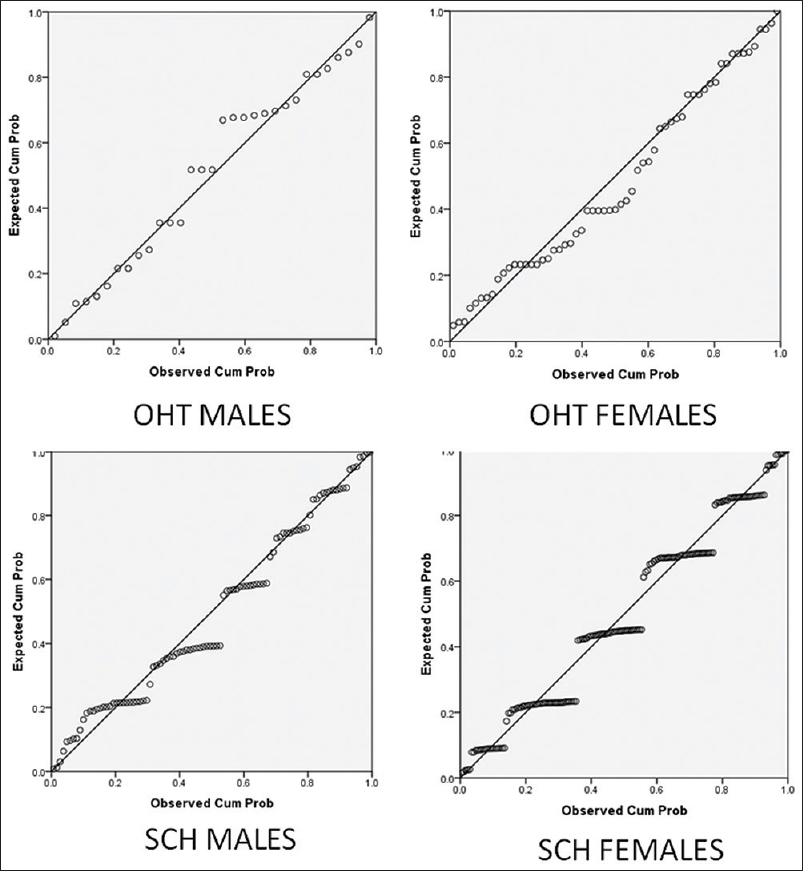
- Residual P-P plot for overt hypothyroid and subclinical hypothyroid groups. (dependent variable is creatinine for variable thyroid-stimulating hormone)
Discussion
In this cross-sectional study, we observed that though the serum creatinine levels were within the normal reference range, it was higher and eGFR was lower significantly in the SCH group when compared to the control group.[312] The overtly hypothyroid group had much higher levels of serum creatinine and lower eGFR when compared to both the groups. This is in accordance with other studies.[31213] It is seen that the creatinine values show a definite trend of increase from the ET cases to the SCHs and finally to the OHT cases as seen in Table 3.
The cause for this difference in the serum creatinine and eGFR in the SCH group and OHT could be due to reduced renal blood flow[12] and tubular function as a result of tissue hypothyroidism. The differences in SCH group and OHT group could explain the severity of the disease. Thyroid hormones influences cardiac output, and hence, in hypothyroid state, there is decreased cardiac output and increased peripheral resistance resulting in an overall decrease in systemic blood volume, thereby decreasing renal blood flow, which can decrease the eGFR. Studies have shown an improvement in eGFR with thyroid replacement therapy indicating that renal dysfunction is predominantly caused by functional changes rather than by permanent histological damage.[3141516]
Thyroid has negligible effect upon the synthesis of creatine from its precursors. In hypothyroid state, creatine is retained in the muscle and the stores of both creatine and phosphocreatine are increased; however, the conversion of creatine to creatinine is uncertain. The rate of excretion of creatinine corresponds to a conversion of 2% of the creatine in the body in 24 h. There is no renal threshold for creatinine. Studies done to find the influence of thyroid on the rate of conversion of creatine into creatinine in hypothyroid state did not show convincing results.[17]
Pharmacologically induced hypothyroidism in rats resulted in a marked reduction in kidney size and creatinine clearance, thus decreasing GFR.[18] Based on the evidences, we can say that the increased levels of creatinine in SCH and OHT groups are due to changes in renal excretion and not the muscle involvement.
We have used calculated eGFR using creatinine-based equation for assessing the changes in renal function. There are many studies that have performed clearance studies using51 Cr-ethylenediaminetetraacetic acid clearance,131 I-hippuran clearance for measuring GFR and have reported that changes in levels of serum creatinine in patients with thyroid disorders do reflect actual changes in GFR.[10]
We have used both MDRD study equation and CKD-EPI equations which are the most widely used IDMS traceable equations for estimating GFR in patients aged 18 and over. Data from various studies showed that the CKD-EPI creatinine equation is more accurate than the MDRD study equation across a wide variety of populations and clinical conditions.[19] We have got a very strong significant positive correlation between eGFR calculated by MDRD and eGFR calculated by CKD-EPI equations [Table 5 and Figure 1]. Hence, either of the equations could be used for estimating eGFR in these groups.
Linear regression analysis using GLM model is shown in Table 6, which shows there is high-adjusted R2 and low residual error in SCH group, where genders are segregated in comparison to OHT group. The positive correlations in the OHT group are found compelling one to work out the regression coefficients of the creatinine values upon the TSH values. It is observed that the TSH values considered gender-wise yield much better regression with the creatinine values in the SCH group than in the control ET group and comparable to the regression values in the OHT group. Hence, regression coefficients have been shown in the Table 7 for OHT and SCH groups but not control ET group. The residual P-P plots shown in Figure 2 demonstrate that the GLM model shows a good fate in respect of the OHT group as well as the SCH group. It can be concluded from these observations that the SCH groups behave biochemically similar to the OHT group.
Some authors have called SCH as mild thyroid failure since the thyroid gland is being stimulated by excess TSH to compensate for maintaining the normal thyroid hormone levels. Furthermore, elevated TSH levels could be a compensatory mechanism to prevent excessive catabolism. It is not associated with increased mortality in elderly patients.[1516] It can be difficult in an individual patient to distinguish a ET subject from one with either mild or overt thyroid disease clinically since all of them have similar constellations of symptoms. Furthermore, when the laboratory reports show a TSH value <10 μIU/L with a normal FT3 and FT4, it is difficult to assess the requirement for treating such patients. There are some studies which have demonstrated evidence of specific neurobehavioral and neuromuscular dysfunction in SCH. A recent study involving subjects from a Chinese population found a higher TSH level in patients with metabolic syndrome compared to that in the nonmetabolic syndrome group suggesting that SCH may be a risk factor for metabolic syndrome.[20] Some authors have found SCH to be an independent and equivalently important risk factor for myocardial infarctions, at least in subjects having TSH values >10 μIU/L.[1521] According to Tsuda et al., SCH is associated with CKD and also TSH is an independent factor for determining renal function in ET individuals.[22]
Conclusion
In this cross-sectional study, we have evaluated one more objective parameter to assess the impact of SCH on the renal system by estimating serum creatinine values and eGFR. This study clearly shows that there is tissue hypothyroidism as manifested by the difference in the creatinine levels and eGFR values in such patients compared to the healthy controls. eGFR calculation by both the formulae has good correlation in the study groups. Hence, either of them can be used for measuring GFR. Furthermore, the linear regression analysis concludes that the TSH values may be used to predict the lower kidney function (higher creatinine values) among the SCH group. This is an important conclusion of this paper that there is probably scope for reversing the decline in renal function among the SCH group. The large samples in this study which shows a definite linear trend of creatinine as a dependent variable on the TSH values is a significant observation. SCH is a common disorder that frequently progresses to OHT. Given the high prevalence of SCH, treatment with thyroid hormone replacement needs to be addressed in an appropriately powered randomized controlled trial. As of now based on the findings of this study, patients with SCH need to be monitored and treated individually based on the symptoms and laboratory investigations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Umesh Dixit for extending his help in statistical analysis.
References
- Subclinical thyroid disease: Scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228-38.
- [Google Scholar]
- Prevalence, clinical and biochemical profile of subclinical hypothyroidism in normal population in Mumbai. Indian J Endocrinol Metab. 2013;17:454-9.
- [Google Scholar]
- Influence of thyroid function on different kidney function tests. Kidney Blood Press Res. 2012;35:9-17.
- [Google Scholar]
- Correlation of creatinine with TSH levels in overt hypothyroidism – A requirement for monitoring of renal function in hypothyroid patients? Clin Biochem. 2012;45:212-4.
- [Google Scholar]
- Interactions between thyroid disorders and kidney disease. Indian J Endocrinol Metab. 2012;16:204-13.
- [Google Scholar]
- Thyroid function and glomerular filtration – A potential for grave errors. Nephrol Dial Transplant. 2005;20:1002-3.
- [Google Scholar]
- Thyroid dysfunction and kidney disease: An update. Rev Endocr Metab Disord. 2017;18:131-44.
- [Google Scholar]
- Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766-72.
- [Google Scholar]
- A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-12.
- [Google Scholar]
- Association of thyroid function with estimated glomerular filtration rate in a population-based study: The HUNT study. Eur J Endocrinol. 2011;164:101-5.
- [Google Scholar]
- The relationship between estimated glomerular filtration rate and thyroid function: An observational study. Ann Clin Biochem. 2008;45:515-7.
- [Google Scholar]
- Correlation between severity of thyroid dysfunction and renal function. Clin Endocrinol (Oxf). 2005;62:423-7.
- [Google Scholar]
- Subclinical hypothyroidism is mild thyroid failure and should be treated. J Clin Endocrinol Metab. 2001;86:4585-90.
- [Google Scholar]
- Preservation of renal function by thyroid hormone replacement in elderly persons with subclinical hypothyroidism. Arch Med Sci. 2016;12:772-7.
- [Google Scholar]
- Effects of thyroid on creatine metabolism with a discussion of the mechanism of storage and excretion of creatine bodies. J Clin Invest. 1946;25:360-77.
- [Google Scholar]
- Renal expression of sodium transporters and aquaporin-2 in hypothyroid rats. Am J Physiol Renal Physiol. 2003;284:F1097-104.
- [Google Scholar]
- The relationship between serum thyrotropin and components of metabolic syndrome. Endocr J. 2011;58:23-30.
- [Google Scholar]
- Risk of coronary heart disease and mortality for adults with subclinical hypothyroidism. JAMA. 2010;304:2481.
- [Google Scholar]
- Relationship between serum TSH levels and intrarenal hemodynamic parameters in euthyroid subjects. Eur J Endocrinol. 2013;169:45-50.
- [Google Scholar]





