Translate this page into:
Evaluation of Xpert MTB/RIF Assay on Stool Samples for the Diagnosis of Pulmonary Tuberculosis among the Pediatric Population
Address for correspondence: Shashank Purwar MD, PhD, Department of Microbiology, All India Institute of Medical Sciences, Bhopal, 462020, Madhya Pradesh, India (e-mail: shashank.microbiology@aiimsbhopal.edu.in).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
Microbiological confirmation of tuberculosis (TB) in pediatric cases is challenging due to its paucibacillary nature and difficulty in specimen collection. This study aimed to validate stool as an alternative sample for the diagnosis of pediatric pulmonary TB via Xpert MTB/RIF (Xpert) assay.
Materials and Methods
This cross-sectional study included 75 pediatric patients up to 10 years of age with signs and symptoms suggestive of TB. From each recruited patient, pulmonary and stool samples were collected in a sterile container. The collected samples were subjected to Ziehl-Neelsen staining, BACTEC MGIT 960 culture (MGIT), Xpert, and in-house multiplex polymerase chain reaction for TB diagnosis.
Results
About 13.33% (10/75) of the pulmonary samples and, of them, 50% (5/75) of the stool samples were positive by Xpert assay. The sensitivity and specificity of Xpert assay with stool and pulmonary samples were 50 (95% confidence interval [CI]: 18.71–81.29%) and 100% (95% CI: 94.48–100%), respectively.
Conclusion
The Xpert assay on stool samples showed limited sensitivity and good specificity in the diagnosis of pulmonary TB. Therefore, it can be proposed as an alternative screening sample to diagnose TB in pediatric cases for which getting a respiratory sample is extremely difficult. However, further studies with greater number of samples and multiple baseline variables are required to support our findings. Strategies to optimize stool Xpert assay should be performed to enhance the sensitivity of this method to detect Mycobacterium tuberculosis in children.
Keywords
Xpert MTB/RIF assay
culture
multiplex PCR
stool sample
pediatric pulmonary tuberculosis
Introduction
Tuberculosis (TB) is a communicable disease and is caused by the bacteria Mycobacterium tuberculosis (MTB) that spread from person to person through the respiratory route.[1–3] TB is the leading cause of death from a single infectious agent ranking even above human immunodeficiency/acquired immunodeficiency syndrome (HIV/AIDS) and also one of the top 10 causes of death worldwide.[1] The global burden of TB is around one-third of the world population.[4] According to the World Health Organization (WHO) Global Tuberculosis report 2020, India is one of the top 20 high TB burden countries in the absolute number of incident cases among the 30 high TB, high TB/HIV, and multidrug-resistant TB burden countries. A common misconception was that children were not severely affected by the global TB epidemic and they rarely develop life-threatening illnesses.[5] However, in the TB endemic area, children most commonly present with an advanced stage of illness. TB is one of the most common causes of morbidity and mortality of children under 5 years of age.[5] According to the WHO Global Tuberculosis Report 2019, the global burden of childhood TB is around 10 to 15% and a high burden country like India is around 25% of the total global burden.[4] In the year 2018, out of total TB cases notified in India around 6% of cases are of children between the age group of 0 and 14 years.[1]
Due to diagnostic uncertainty of the sputum sample culture in children, the diagnosis of pediatric TB mostly depends on clinical assessment and radiological findings, and often the treatment is started based on clinical suspicion.[6] The collection of good quality sputum samples in children is tedious and low bacterial load in the sample makes isolation of the organism difficult.[7,8] It is known that good-quality sputum is 3.8 times more likely to isolate pathogenic bacteria than poor-quality sputum. But it is difficult for children to expectorate sputum. Therefore early morning gastric aspirate alongwith induced sputum and BAL (Bronchoalveolar Lavage) are considered better samples for pediatric tuberculosis diagnosis. Because of the invasiveness of these procedures, they cannot be performed in a primary health center that is an important and primary level of health care facility in India.[5] In addition to the diagnosis of pulmonary TB in children, there is an essential need for the identification of multidrug resistant TB in children and high-risk groups patients to prevent the spread of drug-resistant TB throughout the world.[9,10] There is a need for rapid, reliable, accessible tests and the most easily obtainable type of sample for isolation and identification of MTB.[11] The stool sample is easy to obtain in children, can be collected at the primary health care level without the involvement of invasive procedure, and can be subjected to the culture and for molecular diagnostic methods aiding in the diagnosis of pulmonary TB in children and their referral for appropriate treatment, thus reducing the morbidity and mortality due to TB. In this study, the stool samples will be used for the identification of MTB, as mycobacterial DNA present in the sputum survives the passage through the gastrointestinal tract. The significance of Xpert MTB/RIF in confirmation of TB is assessed by comparing the stool and induced sputum/gastric lavage sample using microscopy, BACTEC MGIT 960 culture, and multiplex polymerase chain reaction (PCR) assay.
Materials and Methods
The study was conducted in a tertiary care health center on children of age group 6 months to 10 years who attended outpatient and inpatient services of the Department of Pediatrics, All India Institute of Medical Sciences, Bhopal (AIIMS, Bhopal) from July 2019 to October 2020. Children satisfying the National Tuberculosis Elimination Program definition of presumptive TB were included in this study. Patients presenting with fever of more than 2 weeks duration and (or) cough of more than 2 weeks duration, loss of appetite, and with recent significant weight loss (10%) were included in this study. Children who were on antitubercular therapy (ATT) and whose guardian was not willing for the study were excluded from the study. In this study, gastric aspirate and induced sputum were considered as the pulmonary sample, as children have difficulty expectorating and tend to swallow the sputum. Single-induced sputum or gastric aspirate and stool sample were collected from patients who were satisfying the inclusion criteria by the pediatrician. The radiological investigation (chest X-ray) was done for all the recruited patients. Also, the immunization status was recorded for all. Samples were processed immediately under the biosafety guidelines. In case of delay, samples were refrigerated at 4°C.
The respiratory sample was subjected to Xpert MTB/RIF according to the manufacturer's instructions[12] (Cepheid, Sunnyvale, California, United States). Approximately, 4 mL of a respiratory sample (gastric aspirate or induced sputum) was taken to which twice the volume, that is, 8 mL, of sample reagent was added. The mixture was mixed, vortexed, incubated, and processed according to the manufacturer's instruction. The respiratory sample was decontaminated and concentrated using N-acetyl-l-cysteine–sodium hydroxide (NALC–NaOH) method before further processing.[13] The decontaminated sample was subjected to Ziehl-Neelsen (ZN) staining, and culture by using BACTEC MGIT 960 liquid culture (►Fig. 1). Stool samples were subjected to stool processing before decontaminating the sample with the NALC–NaOH method. Briefly, one teaspoon (∼1 g) stool sample was mixed with 3 to 4 mL of phosphate buffer saline and mixed thoroughly with a mortar and pestle and filtered through a single layer of gauze.[14] The filtrate was decontaminated with the NALC–NaOH method. Smears were prepared for ZN staining from the decontaminated stool samples. The stool samples were inoculated in BACTEC MGIT 960 system and processed for Xpert MTB/RIF as same as respiratory samples. Samples that showed growth were confirmed for the presence of acid-fast bacilli by acid-fast staining and MPT64 immunochromatographic card test to confirm the presence of MTB in the sample.[15]
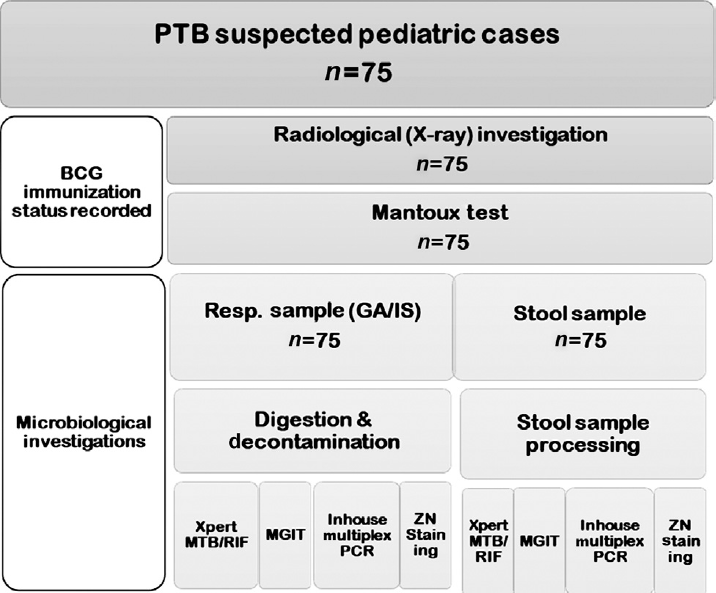
- Workflow of the study. BCG, Bacille Calmette-Guerin; PCR, polymerase chain reaction; PTB, pulmonary tuberculosis; ZN, Ziehl-Neelsen.
Multiplex PCR was done for both respiratory and stool samples. The DNA from MTB suspected samples was extracted and purified using the chloroform: isoamyl alcohol (CI) method. The multiplex PCR that was used in this study was a standardized in-house method with minor modification.[16] The assay primer amplifies the regions of heat shock protein 65 (hsp65), early secretory antigenic target-6 (Esat-6), and internally transcribed sequence (ITS) regions to detect Mycobacterium species, MTB, and Mycobacterium avium complex, respectively.
Statistical Analysis
The BACTEC MGIT 960 positive culture of induced sputum/gastric aspirate was taken as the reference standard. Statistical analysis was performed using Epi Info software.[17] Sensitivity, specificity, positive predictive value, negative predictive value, and Cohan's kappa score were the test of validation calculated in this study. The p-value was calculated using the McNemar test. Diagnostic test characteristics were determined with 95% confidence intervals (CIs).
Results
Total 75 patients were recruited in this study. The mean age of this study population was calculated to be 2.9 (±1.8) years of age, of which 50% of the patients were of 2 years of age. The study population contains 48 males and 27 females. The most predominant clinical presentation noted in the study group was fever that was noted in 46 patients (61%), followed by cough in 33 patients (44%) and weight loss in 27 patients (36%) (►Table 1).
| Patient details | n = 75 |
|---|---|
| Age (years) | Mean: 2.9 (±1.9) |
| Gender | Male: 48 (64%) |
| Female: 27 (36%) | |
| Clinical details | Fever: 46 (61%) |
| Cough: 33 (44%) | |
| Weight loss: 27 (36%) | |
| Radiological findings | Consolidation: 4 |
| Patchy infiltrates: 2 | |
| Miliary pattern: 1 | |
| Immunization status (BCG) | Immunized: 74 (98%) |
| Unimmunized: 1 (2%) | |
| Mantoux test | Positive: 5 (6.6%) |
| Negative: 70 (93%) |
Abbreviation: BCG, Bacille Calmette-Guerin.
The Total Positivity of Xpert MTB/RIF of Stool and Respiratory Samples
Xpert was positive in five stool samples and ten respiratory samples (►Table 2). All the positive Xpert samples were rifampicin sensitive (►Fig. 2). The sensitivity, specificity, positive predictive value, and negative predictive value of stool Xpert, when compared with respiratory sample Xpert, were 50, 100, 100, and 94.489%, respectively, and no statistical significance (p = 0.133) was noted between stool Xpert and respiratory sample Xpert (►Table 3).
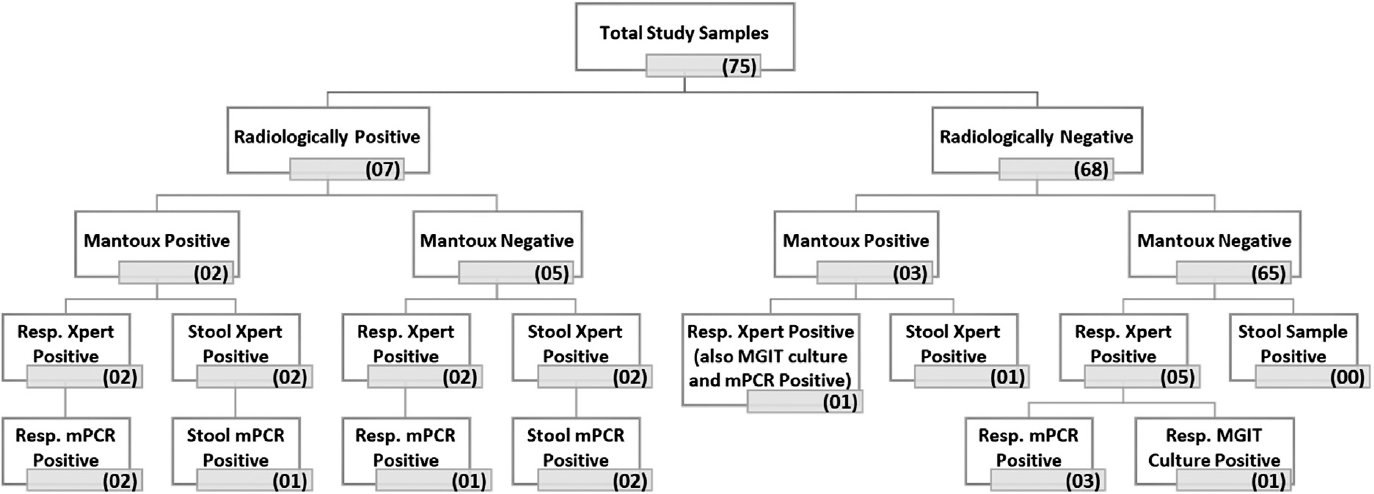
- Results of the microbiological investigation done for stool and respiratory samples of 75 recruited pediatric cases. mPCR, multiplex polymerase chain reaction.
| Sample type | Positive for MTB | Negative for MTB | Total |
|---|---|---|---|
| XpertMTB/RIF | |||
| Respiratory sample | 10 (13.3%) | 65 (86.6%) | 75 |
| Stool sample | 5 (6.6%) | 70 (93.3%) | 75 |
| Culture | |||
| Respiratory sample | 2 (2.6%) | 73 (97%) | 75 |
| Stool sample | 0 | 75 (100%) | 75 |
| Multiplex PCR | |||
| Respiratory sample | 7 (9.3%) | 68 (90.7%) | 75 |
| Stool sample | 3 (4%) | 72 (96%) | 75 |
Abbreviations: MTB, Mycobacterium tuberculosis; PCR, polymerase chain reaction.
| Test method | Reference method | Sensitivity | Specificity | PPV | NPV | Kappa score | p-Value |
|---|---|---|---|---|---|---|---|
| Stool XpertMTB/RIF | Resp. XpertMTB/RIF | 50% (18.71–81.29%) | 100% (94.48–100%) | 100% (56.55–100%) | 92.86% (86.21–97.76%) | 0.687 (0.404–0.971) | 0.133 |
| Stool XpertMTB/RIF | Resp. MGIT culture | 100% (34.24–100%) | 95.89% (88.6–98.59%) | 40% (11.76–6.93%) | 100% (94.8–100%) | 0.554 (0.351–0.757) | 0.248 |
| Stool XpertMTB/RIF | Resp. multiplex PCR | 71.43% (35.89–91.78%) | 100% (94.65–100%) | 100% (56.55–100%) | 97.14% (90.17–99.21%) | 0.8193 (0.597–1.042) | 0.479 |
| Stool Multiplex PCR | Resp. multiplex PCR | 42.86% (15.82–74.95%) | 100% (94.65–100%) | 100% (43.85–100%) | 94.44% (86.57–97.82%) | 0.576 (0.371–0.781) | 0.137 |
Abbreviations: NPV, negative predictive value; PCR, polymerase chain reaction; PPV, positive predictive value.
The Total Positivity of BACTEC MGIT 960 Culture of Stool and Pulmonary Sample
MTB growth was noted in only two pulmonary samples and no stool sample showed growth by BACTEC MGIT 960 culture system (►Fig. 2). The sensitivity, specificity, positive predictive value, and negative predictive value of stool Xpert, when compared with pulmonary sample MGIT, were 100, 95.89, 40, and 100%, respectively, and no statistical significance (p = 0.248) was noted between stool Xpert and MGIT culture of pulmonary samples.
The Total Positivity of Multiplex PCR of Stool and Pulmonary Sample
Seven pulmonary samples and three stool samples were positive by multiplex PCR assay (►Table 2). The sensitivity, specificity, positive predictive value, and negative predictive value of stool multiplex PCR, when compared with multiplex PCR of pulmonary sample, were 42.86, 100, 100, and 94.44%, respectively. However, no statistical significance (p = 0.137) was noted between stool and pulmonary sample multiplex PCR (►Table 3). A comparison of stool Xpert with stool multiplex PCR was also done. The sensitivity, specificity, positive predictive value, and negative predictive value of stool Xpert when compared with stool multiplex PCR were 71.43,100, 100, and 97.14%, respectively (►Table 3). No statistical significance (p = 0.479) was noted.
Discussion
Pediatric pulmonary TB is paucibacillary that often leads to smear-negative on acid-fast bacilli staining, and there is a lack of a practical gold standard test for the diagnosis of the disease. The inability of the preadolescent children to expectorate sputum and low sensitivity (30–40%) of MTB culture makes the diagnosis of pediatric TB difficult.[18] For this reason, the Xpert assay of a stool sample is an appropriate sample in the management of pediatric pulmonary TB as a collection of stool samples is easy and noninvasive compared with other alternatives.[19] Multiple studies assessing the diagnostic utility of Xpert MTB/RIF with stool samples have been noted and studied.
In our study, the analysis of Xpert MTB/RIF with stool sample for the detection of MTB in children with presumptive PTB showed a sensitivity of 100%, CI (34.24–100%) and specificity of 95.89%, CI (88.6–98.59%) as compared with BACTEC MGIT 960 culture findings. Kabir et al[20] have conducted a study on 454 children across four different tertiary care centers in Dakha. They noted 2% of pulmonary samples positive by culture, 2.7% by Xpert, and 6.3% by Xpert Ultra. On the other hand, only two stool samples (i.e., 0.4%) were positive by culture, 11 samples (i.e., 2.5%) by Xpert, and 60 samples (i.e., 13.4%) positive by Xpert Ultra. The sensitivity and specificity of Xpert Ultra on stool were 58.6 and 88.1%, respectively. Xpert on the stool had sensitivity and specificity of 37.9 and 100.0%, respectively. In Kabir et al, stool samples of bacteriologically confirmed cases are included. However, in our study, all patients irrespective of their positivity of pulmonary sample were included. The age group of patients who are included in Kabir et al was of less than 15 years of age. Because of the significant sample size, a good number of children are above the age of 10 years, who can expectorate the sputum sample that in turn improves the quality of the sputum sample and decrease the yield in stool samples.
Sun et al[21] conducted a study in Sichuan province, China, among 141 active TB children and 34 children with respiratory tract infections other than pulmonary TB. The sensitivity and specificity of stool Xpert Ultra (probable and confirmed TB patients) were noted as 60.3 and 97.1%, respectively. Among the confirmed TB group, the sensitivity of stool Xpert Ultra was noted as 85.4%, and among the probable TB group around 47.3%. The sensitivity of 32.6% and specificity of 100% were noted among both probable and confirmed TB cases Xpert stool. This study shows the Xpert Ultra is better than Xpert as the detection limit in Xpert Ultra was much lower compared with Xpert.
In Orikiriza et al,[22] the study outcome revealed sensitivity and specificity of stool Xpert MTB/RIF compared with MGIT culture of the pulmonary sample as 55.6 and 98.2%, respectively. The results were very low compared with our study findings because in this study within 1 week of starting ATT stool samples were collected that could have affected the quality of the paucibacillary sputum sample that in turn the mycobacterial yield in the stool sample. In our study, we have excluded the patients who were on ATT therapy. In another study by LaCourse et al,[23] the sensitivity and specificity were noted as 63 and 98%, respectively. The disconcordance in results may be because, in this study, one stool sample and multiple reference respiratory/gastric aspirate samples were collected that in turn reduced the sensitivity of stool Xpert MTB/RIF significantly.
It is a known fact that HIV alters the course of TB infection. People who are infected with HIV are at increased risk of getting infected with MTB infection.[24] In a study conducted by Chipinduro et al[25] on 222 participants, the positive stool Xpert was noted as 8.2% and there is a slight increase in the number up to 12% among HIV-infected patients. In the study by Chipinduro et al, Xpert MTB/RIF has detected 76.9% of microbiologically confirmed cases of HIV-infected patients, while 50% in the case of non-HIV-infected patients. Similarly, LaCourse et al[23] reported that the sensitivity of stool Xpert MTB/RIF in HIV-infected children was 80%, whereas in non-HIV-infected children it was 33%. However, there was no change in the specificity in both the studied groups. The HIV status of patients enrolled in our study was unknown. The time duration between the collection of pulmonary samples and stool samples was not evaluated in this study and was the limiting factor of the study. Some studies have used two different bowel movements stool samples that were not included in this study. This is a limitation of our study.
Rahman et al[26] tested a total of 152 stool samples, 102 from PTB patients and 50 from non-TB-healthy people. Rahman et al noted stool Xpert positive in 92/102 samples of PTB patients and Xpert negative in all 50 samples of healthy individuals. The sensitivity and specificity of stool Xpert were noted to be 90.2 and 100%. This study has been conducted on adults aged between 20 and 30 years. Surprisingly, high sensitivity and specificity have been seen in stool samples of adults, who can expectorate good quality sputum samples.
In another study, by Moussa et al,[27] the sensitivity and specificity of stool Xpert compared with MGIT culture of the pulmonary sample were 83.33 and 98.73%, respectively. Compared with other studies mentioned, Moussa et al reported good Xpert MTB/RIF results. This may be because in Moussa et al's study, two stool samples were collected from two different bowel movements and they were pooled together and processed. This might be due to the pooling of two different stool samples, collected during two different bowel movements. MTB shedding happens intermittently, by pooling the sample the yield of MTB in the sample increased. However, since to collect and mix both the sample one need to wait for a variable duration time, the delay in processing the sample may influence the outcome.
In a pilot study by Nicol et al,[28] the comparison between stool Xpert MTB/RIF and induced sputum Xpert MTB/RIF was done in 115 children of the 19 to 57 months age group. They reported that pulmonary sample Xpert MTB/RIF was able to detect 64% of children with definite TB and stool Xpert MTB/RIF positivity was around 80%. The recent WHO recommendation for the sensitivity of Xpert MTB/RIF of sputum sample is 65%, and 73% for gastric aspirate, whereas 61% for a stool sample. In our study, we found that stool Xpert MTB/RIF sensitivity is around 55.6%, which is comparable to the pooled sensitivity recommended. The specificity recommended by WHO is 98 to 100%, which is in support of our findings of stool Xpert specificity, that is, 100%.
Limited data are comparing stool Xpert MTB/RIF with multiplex PCR of pulmonary samples. Few studies are there where an in-house PCR method was used. In one study, DiNardo et al[24] performed quantitative PCR on stool samples and compared with it Xpert MTB/RIF. This study was performed on adults and children. In adults, they reported that 70% of samples were positive, and in children, 50% of stool samples were positive for MTB. The multiplex PCR method that was used in this study is an in-house preparation as described by Gopinath and Singh.[16] They have enrolled 145 patients and found that the sensitivity and specificity of multiplex PCR in respiratory samples were 66 and 94%, respectively. Interestingly, we also found comparable, sensitivity and specificity of stool Xpert compared with multiplex PCR as 71 and 100%, respectively. The comparable values indicate that stool Xpert is not a better choice as a diagnostic than multiplex PCR for the pulmonary samples. Furthermore, we also found the sensitivity of stool multiplex PCR as 42%, which was low as compared with the multiplex PCR of pulmonary sample. Cordova et al[8] have used IS6110-PCR on stool samples and reported sensitivity and specificity of 86 and 100%, respectively. Remarkably, our study showed better sensitivity than other studies.
The mycobacterial yield of stool samples could be influenced by multiple factors such as cavitary lesions, where there is a high bacterial load in the sputum, associated HIV infection, disseminated TB, poor immunity, malnutrition, etc.[20] The most commonly used diagnostic modality used for PTB is by time-consuming MTB culture or by direct microscopic observation of acid-fast bacilli, which have low sensitivity and require trained laboratory personnel to examine the stained slides.[19] In such a scenario, Xpert stool is a good testing modality for the diagnosis of pediatric pulmonary TB. However, the diagnostic performance of Xpert MTB/RIF of stool samples in various studies was noted to be not similar. The factor that may be responsible for the difference in the performance of Xpert MTB/RIF from the stool is the different specimen processing method that is being used to remove the PCR inhibitory substance that is present in the sample which is quite common in stool specimen.[29] Therefore, further research needs to be done to identify the optimal method for processing stool samples for Xpert MTB/RIF.
Gebre et al,[30] systematic review and meta-analysis, reviewed and analyzed 11 articles. They included a total of 2,117 participants; most studies collected more than one respiratory sample like gastric aspirate/induced sputum, nasopharyngeal aspirate, and string test and compared with a stool sample. The reference method used against stool Xpert is a culture of the respiratory sample or Xpert of the respiratory sample and sometimes both. The pooled sensitivity and specificity of Xpert stool in bacteriologically confirmed cases are 50% and 99%.
MacLean et al,[31] systemic review and meta-analysis, have assessed the diagnostic accuracy of stool Xpert for the detection of pediatric pulmonary TB. This study showed the pooled sensitivity and specificity of the stool Xpert as 67 and 99%, respectively. Both the meta-analyses have accepted that there is heterogeneity in the reported sensitivity in the included studies. MacLean et al have analyzed stool processing techniques that were used in various studies. Both meta-analyses noted that the variability of the sensitivity of stool Xpert in the included studies is due to the significant variability in the processing of a stool sample.[30,31] Several variables were noted throughout the procedure, namely the difference in the time of collection, method of sampling, number of specimens per patient, the volume of the specimen used for the assay, the difference in reagents that were used, and the difference in the filtration and concentration step.
No stool sample showed growth in culture. This may be attributed to the stringent decontamination and concentration procedure that was done, which probably limits the growth or even kill the MTB present in the stool sample. Therefore, the culture of a stool sample is less sensitive as compared with stool Xpert MTB/RIF.
Thus, our study concludes that stool Xpert MTB/RIF has reasonable diagnostic utility for the diagnosis of pediatric PTB in peripheral and rural health centers where the expertise to collect pulmonary sample is not available and it will help us in diagnosing at least 50% of cases. Patients with high clinical suspicion need to be referred to a higher center for further evaluation.
However, multiple variables like the time difference between the collection of respiratory samples and stool samples, methods of decontamination, and concentration of stool samples need to be evaluated further to increase the yield of mycobacteria in the stool sample.
Authors' Contributions
J.S. was involved in performing the experiments, analysis of data, and writing and reviewing the manuscript. R.M. performed the experiments. P.S. edited and reviewed the manuscript. A.K. Maurya reviewed the manuscript. A.G.M. contributed to analysis of data and review of manuscript. S.M. was involved in analysis and interpretation of data and review of manuscript. S.P. was involved in conceptualization of the study, analysis, and interpretation of data, writing, and review of the manuscript. S.S. contributed to conceptualization of the study, interpretation of data, and review of the manuscript.
Ethical Approval
Ethical clearance for the study was obtained from the Institutional Human Ethics Committee (IHEC), AIIMS, Bhopal, Madhya Pradesh, India (Approval No: IHEC-LOP/2020/MD0055).
Acknowledgment
We would acknowledge Dr. Milind Shinde, Department of Pediatrics, AIIMS, Bhopal, Madhya Pradesh, India, for helping us with sample collection. The authors would also like to acknowledge Mr. Mukesh Patel, Department of Microbiology, AIIMS, Bhopal, Madhya Pradesh, India, for assisting in the processing of samples. We are grateful to Cepheid India Pvt. Ltd. for providing the Xpert MTB/RIF cartridge required for the study.
Conflict of Interest
None declared.
References
- World Health Organization, WHO. Accessed August 8, 2022 at: http://www.who.int/tb/publications/global_report/en/
- [Google Scholar]
- Centers for Disease Control and Prevention. Accessed August 8, 2022 at: https://www.cdc.gov/tb/topic/basics/default.htm
- [Google Scholar]
- History of pulmonary tuberculosis. Thorac Surg Clin. 2019;29(01):1-17.
- [CrossRef] [PubMed] [Google Scholar]
- A novel sample processing method for rapid detection of tuberculosis in the stool of pediatric patients using the Xpert MTB/RIF assay. PLoS One. 2016;11(03):e0151980.
- [CrossRef] [PubMed] [Google Scholar]
- Childhood tuberculosis: an emerging and previously neglected problem. Infect Dis Clin North Am. 2010;24(03):727-749.
- [CrossRef] [PubMed] [Google Scholar]
- Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(06):451-461.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of Xpert MTB/RIF testing for rapid diagnosis of childhood pulmonary tuberculosis in children by Xpert MTB/RIF testing of stool samples in a low resource setting. BMC Res Notes. 2017;10(01):473. DOI: 10.1186/s13104-017-2806-3
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of molecular tools for detection and drug susceptibility testing of Mycobacterium tuberculosis in stool specimens from patients with pulmonary tuberculosis. J Clin Microbiol. 2010;48(05):1820-1826.
- [CrossRef] [PubMed] [Google Scholar]
- What does it take to satisfy Koch's postulates two centuries later? Microbial genomics and Propionibacteria acnes. J Invest Dermatol. 2013;133(09):2141-2142.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculosis: new aspects of an old disease. Int J Cell Biol. 2011;2011 403623 DOI: 10.1155/2011/403623
- [CrossRef] [PubMed] [Google Scholar]
- Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537-544.
- [CrossRef] [PubMed] [Google Scholar]
- Accessed August 8, 2022 at: https://www.finddx.org/wp-content/uploads/2016/02/mgit_manual_nov2006.pdf
- Diagnostic accuracy of Xpert Mtb/Rif assay in stool samples in intrathoracic childhood tuberculosis. J Tuberc Ther. 2018;3(02):115-119.
- [Google Scholar]
- Utility of MPT64 antigen detection for rapid confirmation of mycobacterium tuberculosis complex. J Glob Infect Dis. 2015;7(02):66-69.
- [CrossRef] [PubMed] [Google Scholar]
- Multiplex PCR assay for simultaneous detection and differentiation of Mycobacterium tuberculosis, Mycobacterium avium complexes and other Mycobacterial species directly from clinical specimens. J Appl Microbiol. 2009;107(02):425-435.
- [CrossRef] [PubMed] [Google Scholar]
- Accessed August 8, 2022 at: https://www.openepi.com/Menu/OE_Menu.htm
- AIDS orphans and vulnerable children in India: problems, prospects, and concerns. Soc Work Public Health. 2012;27(03):205-212.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert MTB/RIF system. J Clin Microbiol. 2011;49(04):1202-1205.
- [CrossRef] [PubMed] [Google Scholar]
- Variations in rifampicin and isoniazid resistance associated genetic mutations among drug naïve and recurrence cases of pulmonary tuberculosis. Int J Infect Dis. 2021;103:56-61.
- [CrossRef] [PubMed] [Google Scholar]
- Use of Xpert MTB/RIF Ultra assay on stool and gastric aspirate samples to diagnose pulmonary tuberculosis in children in a high-tuberculosis-burden but resource-limited area of China. Int J Infect Dis. 2022;114:236-243.
- [CrossRef] [PubMed] [Google Scholar]
- Xpert MTB/RIF diagnosis of childhood tuberculosis from sputum and stool samples in a high TB-HIV-prevalent setting. Eur J Clin Microbiol Infect Dis. 2018;37(08):1465-1473.
- [CrossRef] [PubMed] [Google Scholar]
- Stool Xpert MTB/RIF and urine lipoarabinomannan for the diagnosis of tuberculosis in hospitalized HIV-infected children. AIDS. 2018;32(01):69-78.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic and treatment monitoring potential of a stool-based quantitative polymerase chain reaction assay for pulmonary tuberculosis. Am J Trop Med Hyg. 2018;99(02):310-316.
- [CrossRef] [PubMed] [Google Scholar]
- Stool Xpert® MTB/RIF test for the diagnosis of childhood pulmonary tuberculosis at primary clinics in Zimbabwe. Int J Tuberc Lung Dis. 2017;21(02):161-166.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of Xpert MTB/RIF assay for detection of Mycobacterium tuberculosis in stool samples of adults with pulmonary tuberculosis. PLoS One. 2018;13(09):e0203063.
- [CrossRef] [PubMed] [Google Scholar]
- Gene Xpert for direct detection of mycobacterium tuberculosis in stool specimens from children with presumptive pulmonary tuberculosis. Ann Clin Lab Sci. 2016;46(02):198-203.
- [Google Scholar]
- Xpert MTB/RIF testing of stool samples for the diagnosis of pulmonary tuberculosis in children. Clin Infect Dis. 2013;57(03):e18-e21.
- [CrossRef] [PubMed] [Google Scholar]
- Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat Rev Microbiol. 2018;16(02):80-90.
- [CrossRef] [PubMed] [Google Scholar]
- Variable diagnostic performance of stool Xpert in pediatric tuberculosis: a systematic review and meta-analysis. Open Forum Infect Dis. 2020;8(08):ofaa627.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of stool Xpert MTB/RIF for detection of pulmonary tuberculosis in children: a systematic review and meta-analysis. J Clin Microbiol. 2019;57(06):e02057-e18.
- [CrossRef] [PubMed] [Google Scholar]






