Translate this page into:
Evidence Based Solving Approach in Diagnosis of Primary Hyperparathyroidism with Oral Manifestations: Report of Three Unusual Cases
Address for correspondence: Dr. Harkanwal Preet Singh, E-mail: hkps0320@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Primary hyperparathyroidism is a common condition that affects 0.3% of the general population in which excessive production of PTH is there. With changing trends it is diagnosed early and asymptomatically with the improvements in routine biochemical tests and radiological procedures. The late bony complications of the disease have therefore started to decline rapidly. The mandible is the predominantly affected site in the maxillofacial area. Maxillary involvement is rare. Here, we reported series of three cases of 30-40-year-old women with osteolytic lesions and bone resorption in maxilla or mandible. Two of presented cases demonstrated evidence of lesions in both mandible and maxilla which is a very rare event. A thorough diagnostic work-up emphasizing on biochemical and radiographic investigations were discussed. We highlighted the role of endocrinologist, oral and maxillofacial surgeons, general practitioner dentists, and radiologists in diagnosing and managing such patients.
Keywords
Endocrinologist
hyperparathyroidism
mandible
osteolytic lesions
INTRODUCTION
Parathyroid glands secrete parathyroid hormone (PTH) involved in regulating the metabolism of calcium and phosphorus. PTH plays an important role in tooth development and bone mineralization and increases bone resorption.[1] Excessive secretion of PTH is termed as hyperparathyroidism (HPT) which is an endocrine condition that occurs in three categories: Primary, secondary and tertiary. Primary HPT (PHPT) occurs with a hyperfunction of one or more parathyroids, causing an increase of parathyroid hormone (PTH) secretion and resulting in hypercalcemia. Secondary HPT, normally related to chronic renal failure, occurs with a decrease of vitamin D production or with hypocalcemia, making the glands to produce a high quantity of PTH. Tertiary HPT can occur in a small number of patients, when the parathyroids activity turns autonomous and excessive; leading to hypercalcemia.[2] It is the third most common endocrine disorder affecting 0.3% of the general population, 1-3% of postmenopausal women and a total population incidence of 21.6 cases per 100,000 person years. PHPT usually occurs as the result of sporadic parathyroid adenomas or carcinomas, but can also be seen in association with rare genetic syndromes and metabolic diseases.[34]
Worldwide, the presentation of PHPT has changed from a symptomatic to an asymptomatic disease. In India, however, asymptomatic presentation is virtually unheard of. Even the symptomatic disease is picked up late after a series of management for fractures and renal stones by the orthopedic surgeons and the urologist.[4] Because of the progress in routine biochemical screening, today HPT can be diagnosed much earlier, mainly in the asymptomatic stage, whereas it was generally diagnosed as an overt disease with predominantly skeletal manifestations in the 1970s.[5] HPT with oral manifestation may pose great challenge to diagnosis by oral physician. For early diagnosis of mild and asymptomatic cases of PHPT, more systematic, methodological, and multidisciplinary approach should be chosen for diagnosing and managing such cases. We hereby reported series of three cases of HPT with oral manifestation emphasizing on systematic diagnostic approach.
CASE REPORTS
Case 1
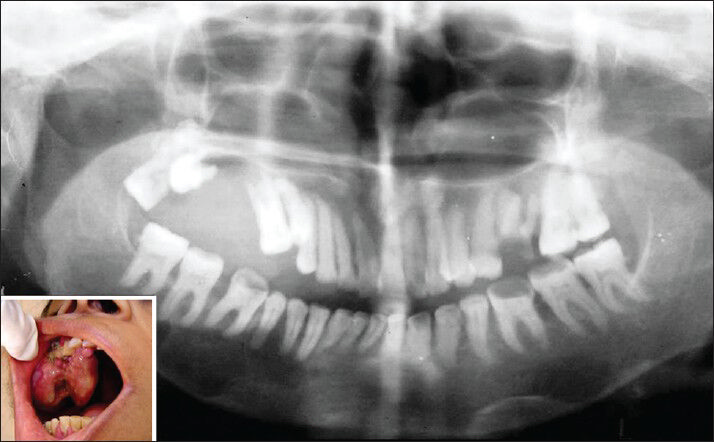
A 32-year-old female patient reported with complaint of swelling over the front right side of the lower jaw since 2 months with history of a small painful swelling over the chin region which gradually increased to the present size [Figure 1]. Patient also gave history of joint pain, backache, and weakness and renal stones 2 years back.

- Extraoral photograph showing swelling over the mental and right body of mandible (inset) and orthopantomograph showing irregular radiolucency in the mandible i.r.t 35-45 region
Extraoral examination revealed a well-defined, soft to firm and tender swelling over the mental and right body of mandible of 6 × 4 cm in size which extended anteroposteriorly from left parasymphysis to the right mandibular body and superoinferiorly from the level of vermilion border of lower lip to the submental region [Figure 1; inset]. Detailed intraoral examination revealed obliteration of labial vestibule i.r.t 41-45 region with Grade 1 mobility of involved teeth. Radiographic examination revealed irregular radiolucency with ill-defined margins seen in the mandible extending from 35-45 region. Generalized rarefaction of the trabeculae and altered trabecular pattern was also seen along with generalized loss of lamina dura [Figure 1]. Full mouth intraoral periapical (IOPA) survey revealed generalized loss of lamina dura. Computed tomography (CT) scan examination revealed gross bone destruction with soft tissue mass.
Case 2
A 40-year-old female patient presented with swelling in the right maxillary region since 6 months with history of swelling in the same region 5 years back and thereafter swelling was excised. A small sized swelling recurred in the same region which gradually increased to the present size. Patient also gave the history of 5-6 kg weight loss in the past few months and felt anorexic and depressed.
Extraoral examination revealed a firm, nontender round swelling, 6 cm in diameter in right maxillary region which extended anteroposteriorly from ala of nose to 2 cm medial to tragus of ear and superoinferiorly 2 cm below infraorbital margin to 1 cm below corner of the mouth.
Intraoral examination revealed a firm, nontender, nonpulsatile reddish brown growth of size 5 × 5 cm extending buccally and palatally i.r.t 12-18 region. Displacement and mobility of 13,14, and 15 pus discharge with 12, 13, and 14 was noticed [Figure 2; inset].
- Intraoral photograph revealing a growth extending buccally and palataly i.r.t 12-18 region (inset) and orthopantomograph showing resorption of right maxillary alveolar region with multiple cyst-like radiolucent areas in right side of body of the mandible
IOPA radiograph revealed loss of lamina dura and cyst-like lesions i.r.t 45, 46, 47. Orhtopantomogram (OPG) revealed a soft tissue mass causing resorption of right maxillary alveolar region with displacement of regional teeth. Multiple cyst-like radiolucent areas in right side of body of the mandible and thinning of inferior border of mandible was observed. Generalized loss of lamina dura was also observed [Figure 2]. CT scan was also carried out which revealed tumor causing destruction of the alveolar bone in right maxilla and lytic lesion involving the left body of mandible [Figure 3].
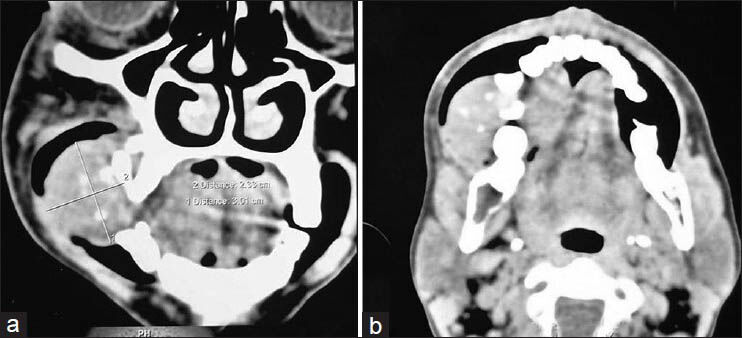
- Computed tomography scan showing: (a) destruction of the alveolar bone in right maxilla and lytic lesion involving the left body of mandible and; (b) lytic lesion involving the left body of mandible
Case 3
A 38-year-old female complained of swelling in anterior maxillary region since 2 months. She gave history of swelling in mandibular right posterior region 3 years back which was diagnosed as central giant cell granuloma and removed surgically. Two months later left posterior teeth were extracted due to mobility. No significant contributory medical history was there. Intraoral examination revealed a firm, nontender, nonpulsatile swelling extending from 23 to 26 with displacement of 23, 24, and 25 with grade 1 mobility [Figure 4; inset]. Patient was known case of hypertension since 2 years.
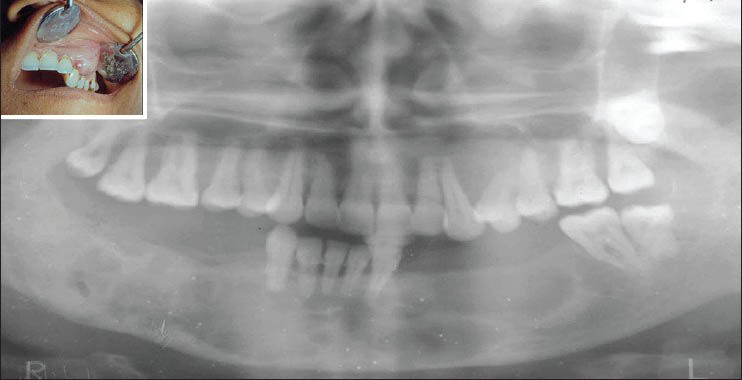
- Intraoral photograph showing swelling on left side of maxilla (inset) and orthopantomograph showing resorption of left posterior maxillary alveolar region with multiple, radiolucent, cyst-like lesions involving whole of the body of mandible
Radiographic examination revealed a soft tissue mass causing resorption of left posterior maxillary alveolar region with displacement of 23 and 24. Multiple, radiolucent, cyst-like lesions involving whole of the body of mandible and thinning of inferior border of mandible was observed as incidental finding [Figure 4]. CT scan was also performed which revealed osteolytic lesions in the whole body of mandible.
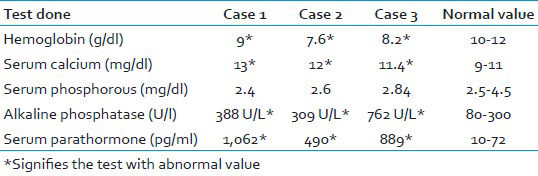
These clinicoradiographic findings raised the suspicion of HPT. So further investigations in the form of radiographic skeletal survey, biochemical tests [Table 1], fine needle aspiration cytology histopathology, and nuclear imaging was carried out in all the three cases.
The patients were thereafter subjected to incisional biopsy and histopathologic examination revealed numerous capillaries and yellowish brown pigmentation due to hemosiderin deposition along with innumerable multinucleated giant cells [Figure 5]. Based on these clinicoradiographic, histopathological and biochemical evidences confirmative diagnosis of HPT was given. All patients were treated surgically followed by medicinal therapy. Regular follow up of patients were done with significant improvement without any reported recurrence.
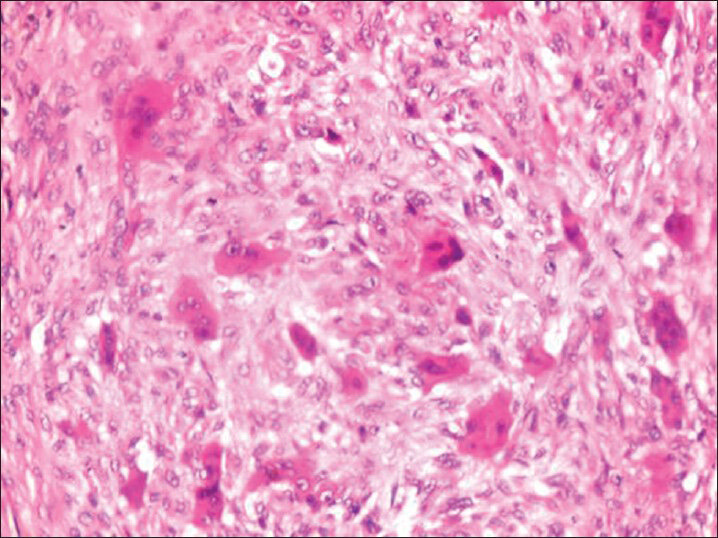
- Photomicrograph (×40) showing multinucleated giant cells in fibrocellular stroma
DISCUSSION
Sylvanus (1743) was the first to diagnose HPT. Recklinghausen (1891) is credited with the first description of the associated bone changes known as osteitis fibrosa cystica.[6]
Fall in the extracellular calcium concentration stimulates PTH secretion which subsequently increases renal calcium reabsorption. It further stimulates osteoclast activating factors such as interleukin-6 from osteoblasts and promotes bone resorption and thereby PTH helps to restore any tendency to hypocalcemia. Current understanding of calcium homeostasis has revealed another view point that when extracellular calcium binds to the calcium receptor in the parathyroid cell, PTH secretion and parathyroid cell growth are inhibited. At the kidney, this interaction between calcium and the calcium receptor inhibits the 1-hydroxylation of 25-hydroxyvitamin D. Calcium affects the thyroid C cells, stimulating calcitonin release, and in bone may potentially regulate bone resorption.[7]
In this lesion, excessive production of PTH cause conversion of potentially osteogenic cell from osteoblast to osteoclast, with bone resorption exceeding the formation of new osseous tissue resulting in bone resorption with fibrous replacement of the marrow and thinning of the cortex. Brown tumors are focal lesion found within these areas of resorption.[67] Prevalence of brown tumors is 0.1% as reported in literature. They are reported to occur in 4.5% of patients with PHPT and in 1.5-1.7% with secondary disease.[6]
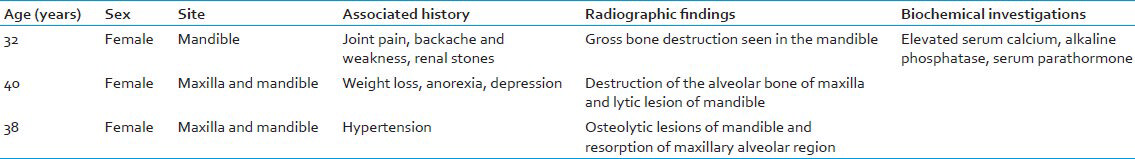
The clinical findings of HPT have been described by Jackson and Frame as the tetrad of bones, stones, abdominal groans, and psychic moans with fatigue overtones and these features were also appreciated in our cases. The condition occurs most commonly in women with a peak incidence between 40 and 50 years. It occurs more often in the mandible compared to the maxilla.[8] Case 1 of series manifested in mandible, whereas other two cases occurred in both maxilla and mandible which is very rare event. The most significant point about the case 2 and 3 here is the simultaneous occurrence of lesion in the maxilla and mandible. According to published articles in English literature, authors have determined that only six such cases of simultaneously occurrence of HPT in the maxilla and the mandible have been reported. All the three cases occurred in women of age ranged between 30 and 40 years which fall well with data given in literature [Table 2].
The bony changes in HPT are identical, whether the cause is primary, secondary, or tertiary. The classic radiographic features are as follows: (1) Subperiosteal bone resorption. (2) Generalized demineralization. (3) Localized lytic bone lesions. (4) Metastatic calcification of soft tissue. The imaging modalities commonly used in patients with PHPT include ultrasonography (USG), magnetic resonance imaging, CT, and the Sestamibi scan, which aids in localization of abnormal gland.[9] In series of our cases; OPG, CT scan, and USG were used as investigative modalities which showed osteolytic lesions with alveolar bone resorption along with generalized loss of lamina dura [Table 2].
Histological and radiographically, it is very similar to the other giant cell lesions (true giant cell tumor, reparative giant cell granuloma, cherubism, and aneurysmal bone cyst).[5] On clinical examination and using only routine panoramic radiography, the lesions may resemble osteosarcoma, bone metastases of a carcinoma, multiple myeloma, Langerhan's cell histiocytosis, Paget's disease, osteomyelitis, or osteonecrosis secondary to bisphosphonate therapy. The differential diagnosis is based on the clinical findings and the presence of HPT, which is confirmed with biochemical tests including PTH level.[5] Multiple giant cell lesions of oral cavity could be one of the earliest manifestation of PHPT. In third case of our series, patient was initially misdiagnosed as central giant cell granuloma and treated for same, but after 3 months lesion recurred and involved other jaw also.
Latter on proper clinical, radiographic, and biochemical investigations were carried out which confirmed diagnosis of HPT. Various laboratory investigations are key to diagnosis of such entities and therefore we emphasize on thorough understanding of pathophysiologic basis of such lesions. PHPT is diagnosed when intact PTH levels are elevated or at the high end of the normal range in the setting of elevated total or ionized calcium levels. Because about 40% of calcium is bound to albumin, serum calcium levels should be corrected for albumin. Repeated measurements (usually three) of calcium and PTH should be taken before establishing the diagnosis of PHPT.[1011] In our cases reported here, constant monitoring of serum calcium and PTH were carried out and were found out to be elevated [Table 1]. In the third International Workshop on PHPT it was concluded that reference ranges should be established for serum PTH in vitamin D-replete healthy individuals, DNA sequence testing can be useful in familial HPT or hypercalcemia, normocalcemic PHPT is a variant of the more common presentation of PHPT with hypercalcemia and serum 25-hydroxyvitamin D levels should be measured and, if vitamin D insufficiency is present, it should be treated as part of any management course.[12]
Recently, a comprehensive analysis of 26 primary hyperparathroid patients by Rai et al., revealed that loss of lamina dura, ground glass appearance, and mandibular cortical thickness reduction are not an uncommon findings in PHPT and these are significantly correlated with elevated parathormone and alkaline phosphatase.[13]
Excluding other causes of hypercalcemia is necessary before confirming the diagnosis. An accurate history of skeletal fragility, renal disease, calcium intake, symptoms suggestive of malignancy, thyroid disease, adrenal insufficiency, previous bariatric surgery, and pancreatic disease; and a thorough review of medications including thiazide diuretics and lithium are necessary. After exclusion of malignancy, granulomatous diseases, or other causes of hypercalcemia; PHPT can be confirmed. This is particularly important if the serum PTH is not elevated.[11]
Complication of HPT is a chronic renal disease (CRD), which is a multifactorial syndrome characterized by progressive and irreversible loss of renal function. The most common complication of CRD includes secondary HPT, hypertension, immunosuppression, infection, anemia, bleeding disorder, and metatstatic calcifications.[14] In the cases reported here, case 3 also revealed history of hypertension which further suggests a complication of HPT. The most common sequel is renal calcification which was appreciated in our case 1.
According to the National Institute of Health Consensus Conference 2002 surgery is recommended for all patients with symptomatic PHPT, whereas asymptomatic individuals must also be treated surgically when patients are younger than 50 years, have severe hypercalcemia, markedly reduced creatinine clearance, and/or profound osteopenia.[15] Limited data is available on the surgical approach to PHPT in most of the literature from India. The approach has ranged from bilateral conventional neck exploration and unilateral neck exploration to focused parathyroidectomy.[416] Although surgery is the definitive cure, not all patients undergo undergo parathyroidectomy. Some patients have comorbid conditions that preclude surgical treatment and many patients seek alternative therapies. Triantafillidou et al., stated that the significant bone disease can be prevented or reduced by medical treatment, such as calcium carbonate, vitamin D, and aluminum hydroxide antacids for hyperphosphatemia. In contemporary medical treatment alternatives, lanthanum carbonate, sevelamer, and calcimimetics such as cinacalcet are more commonly preferred.[17] Recurrent PHPT has ranged from 0 to 4.16% and persistent PHPT between 0 and 2.7%.[4]
CONCLUSION
This theatrical entity therefore highlights the importance of early diagnosis of HPT with a methodological assessment of all the radiographic, biochemical, and histopathological parameters. By this series of cases we emphasize the interdisciplinary approach general practitioner dentists, oral and maxillofacial surgeons, endocrinologists and radiologists in early diagnosis and management of HPT patient which could be helpful in reducing the latter complications and fatal results.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Dental management of patients with endocrine disorders. J Clin Exp Dent. 2010;2:e196-203.
- [Google Scholar]
- Primary, secondary, and tertiary hyperparathyroidism. Otolaryngol Clin North Am. 2004;37:701-13.
- [Google Scholar]
- Primary hyperparathyroidism. Incidence, morbidity, and potential economic impact in a community. N Engl J Med. 1980;302:189-93.
- [Google Scholar]
- Systematic review of primary hyperparathyroidism in India: The past, present, and the future trends. Int J Endocrinol 2011 2011 921814
- [Google Scholar]
- Brown tumour of the maxilla and mandible: A rare complication of tertiary hyperparathyroidism. Dentomaxillofac Radiol. 2009;38:53-8.
- [Google Scholar]
- Bilateral maxillary brown tumors in a patient with primary hyperparathyroidism: Report of a rare entity and review of literature. J Oral Maxillofac Pathol. 2011;15:56-9.
- [Google Scholar]
- Primary hyperparathyroidism: Pathophysiology and impact on bone. CMAJ. 2000;163:184-7.
- [Google Scholar]
- Simultaneous maxillary and mandibular brown tumors in secondary hyperparathyroidism: A case report. Dent Res J. 2008;5:41-5.
- [Google Scholar]
- Extensive skeletal manifestations in a case of primary hyperparathyroidism. Indian J Radiol Imaging. 2002;12:267-70.
- [Google Scholar]
- Hyperparathyroidism revisited-Old wine in new bottles! Indian J Endocrinol Metab. 2011;15:194-7.
- [Google Scholar]
- Primary hyperparathyroidism: Update on presentation, diagnosis, and management in primary care. Can Fam Physician. 2011;57:184-9.
- [Google Scholar]
- Diagnosis of asymptomatic primary hyperparathyroidism: Proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94:340-50.
- [Google Scholar]
- Oro-mandibular manifestations of primary hyperparathyroidism. Indian J Dent Res. 2012;23:384-7.
- [Google Scholar]
- Primary hyperparathyroidism: A rare endocrinopathy in children. Two case reports. Endocrinol Pol. 2011;62:346-50.
- [Google Scholar]
- Brown tumors of the jaws associated with primary or secondary hyperparathyroidism. A clinical study and review of the literature. Am J Otolarygol. 2006;27:281-6.
- [Google Scholar]





