Translate this page into:
Hematological parameters of human immunodeficiency virus positive pregnant women on antiretroviral therapy in Aminu Kano Teaching Hospital Kano, North Western Nigeria
Address for correspondence: Dr. Ibrahim Abdulqadir, Department of Haematology and Blood Transfusion, UDUTH, Sokoto, Nigeria. E-mail: abuahmad.ia@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
CONTEXT:
Human immunodeficiency virus (HIV) scourge continues to affect young women within the reproductive age group and pregnancy is a recognized indication for the use antiretroviral (ARV) drugs among HIV-positive women.
AIMS:
The aim is to determine the combined effect of pregnancy, HIV and ARV drugs on the hematological parameters of the pregnant women.
SETTINGS AND DESIGN:
This was a comparative cross-sectional study conducted among 70 each of HIV-positive and negative pregnant women.
SUBJECTS AND METHODS:
Bio-demographic and clinical data were extracted from the client folder and 4 ml of blood sample was obtained from each participant. Full blood count was generated using Swelab automatic hematology analyzer while reticulocyte count and erythrocyte sedimentation rate (ESR) were conducted manually.
STATISTICAL ANALYSIS USED:
Data analysis was performed using SPSS version software 16 while P < 0.05 was considered statistically significant.
RESULTS:
Pregnant women with HIV had statistically significant lower hematocrit and white blood cell (WBC) and higher ESR than pregnant women without HIV (P < 0.000). There was no statistically significant difference between the two groups in terms of platelet and reticulocyte (P > 0.05). However, among HIV positive pregnant women, those with CD4 count <350/μL had statistically significant lower WBC and lymphocyte count than those with CD4 count ≥350/μL (P < 0.05), whereas, those on zidovudine (AZT)-containing treatment had statistically significant lower hematocrit and higher mean cell volume than those on non-AZT-containing treatment (P < 0.05), but there was no statistically significant difference in any of the hematological parameters (P > 0.050) between women on first- and second-line ARV regimens.
CONCLUSIONS:
There is a significant difference in terms of hematological parameters between HIV-positive and HIV-negative pregnant women in this environment.
Keywords
Antiretroviral drugs
hematological parameters
human immunodeficiency virus
Kano
pregnant women
Introduction
Human immunodeficiency virus (HIV) epidemic continues to affect young women within the reproductive age with a prevalence of 4.1% among pregnant women in Nigeria.[1] From 2010, pregnancy has become an indication for the use of triple antiretroviral (ARV) drugs for prophylaxis or therapy in HIV-positive women in Nigeria.[2] Both pregnancy, HIV and ARV drugs were unequivocally shown to affect hematological parameters of the individual.[345678] Many studies were carried out to access the effect of these on the hematological parameters.[91011] However, they were mainly done in isolation, and there is a paucity of data on the combined effect of HIV, ARV drugs and pregnancy on the hematological parameters, especially in this part of the country. Thus, this study aimed to determine the hematological parameters of HIV pregnant women on ARV therapy in Aminu Kano Teaching Hospital (AKTH), Kano state, North Western, Nigeria.
Subjects and Methods
This was a comparative cross-sectional study conducted among 70 each of HIV-positive and negative pregnant women from October 2014 to December 2016. Participants were all consenting pregnant women admitted for delivery at the labor ward of the AKTH, Kano. Women with Sickle cell disease, hypertension, liver, renal, or endocrine diseases were excluded from the study. Also excluded were all pregnant women who seroconvert during index pregnancy and were not on ARV drugs for at least 3 months.
Bio-demographic and clinical data of the participants were extracted from their case folder and 4 ml of blood were aseptically collected from the antecubital vein of the pregnant women with Vacutainer into K2 ethylenediaminetetraacetic acid bottle. Sample was processed within 4 h of collection. Full blood count was generated using Swelab 3-part automated differential Coulter while reticulocyte count and erythrocyte sedimentation rate (ESR) were done manually. HIV serologic test was done with DetermineTM HIV-1/2 Ag/Ab Combo.
In this study, HIV-positive means any pregnant woman who was tested positive to HIV antibodies and confirmed by a second HIV test method and is aware of her status, whereas HIV negative means any pregnant woman who was tested negative for HIV antibodies and is aware of her status. Seroconvert means any pregnant woman who had HIV negative test earlier during index pregnancy and now tested positive to p24 antigen, HIV-1 or HIV-2 antibodies or both. HIV positive pregnant women were grouped based on CD4 count (CD4 ≥350/μL and <350/μL), ARV regimen ( first-line and second-line), and type of ARV (AZT based and non-AZT based).
Informed written consent was obtained from each participant, and the study was approved by the Research Ethics Committee of the Hospital.
Data generated were analyzed using Statistical Programme (SPSS 20, Statistical Package for the Social Sciences IBM Corp. Released 2011 version 20. Armonk, NY) and result was presented as mean (±standard deviation). Independent sample t-test was used to compare means of the hematological parameters, and the value of P ≤ 0.05 was considered to be statistically significant.
Results
The HIV-positive women had a mean age of 29.06 ± 6.87 years, systolic blood pressure (SBP) of 113.57 ± 10.50 mmHg, diastolic blood pressure (DBP) of 65.86 ± 6.70 mmHg, and booking packed cell volume (PCV) of 32.79 ± 2.47%, whereas the corresponding mean values for HIV-negative women were 28.34 ± 5.94 years, 115.14 ± 10.32 mmHg, 66.00 ± 6.68 mmHg, and 33.63 ± 2.41%, respectively. There was no statistically significance difference between the two groups in terms of age (P = 0.230), SBP (P = 0.886), DBP (P = 0.980), and PCV at booking (P = 0.829).
The mean hematocrit and white blood cell (WBC) for the HIV-positive pregnant women were found to be significantly lower than those of HIV-negative pregnant women, whereas the ESR of HIV-positive pregnant women were found to be significantly higher than that of HIV-negative pregnant women; P < 0.05. Other hematological parameters and results of comparison between these parameters for HIV and non-HIV pregnant women are depicted in Table 1.
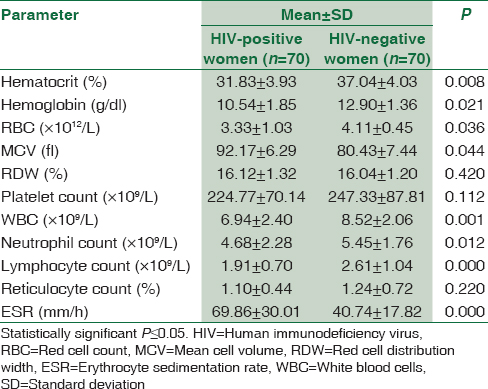
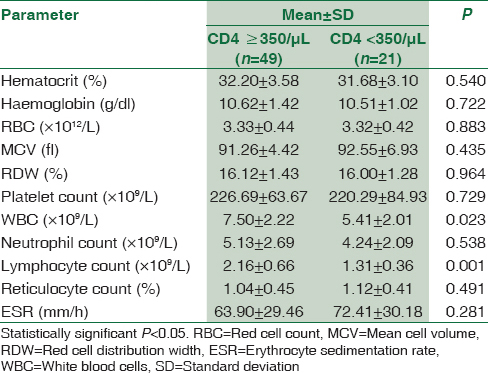
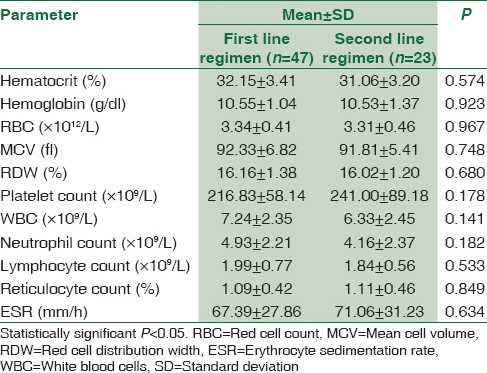
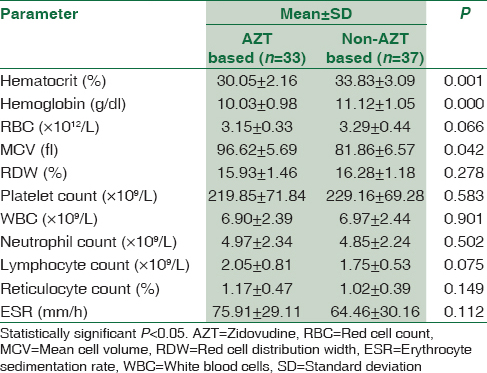
Pregnant women with HIV were grouped according to their CD4 count, ARV regimen and type of ARV and their mean hematological parameters were compared using independent sample t-test, and the result is presented in Tables 2-4. HIV-positive women with CD4 ≥350/μL were found to have significantly higher WBC and lymphocyte count than those with CD4 <350/μL; P < 0.05. There was no significant difference in any of the hematological parameters between HIV-positive women on first-line ARV and second-line ARV regimens; P ≥ 0.05. HIV-positive women on AZT-containing ARV treatment were found to have significantly lower hematocrit and higher mean cell volume (MCV) than those on non-AZT-containing ARV treatment; P < 0.05. The results of comparison between hematological parameters of HIV-positive pregnant and CD4 counts, ARV regimen and type of ARV are depicted in Tables 2-4, respectively.
Discussion
The low hematocrit among HIV-positive women in relation to their HIV-negative counterpart in this study was similar to the findings of many studies among HIV pregnant women.[121314] This could result from either decrease production or increase destruction of red blood cell. However, the mild anemia among HIV-positive pregnant women seen in the study can be ascribed to the effect of highly active antiretroviral therapy (HAART) since most of the hematological abnormalities in HIV are known to be prevented, ameliorated, or corrected by the use of HAART.[1516] The macrocytosis among HIV positive women that is more pronounced in those on AZT-based ARV drugs, in this study was also reported by other studies and could be related to the number of women taking zidovudine (AZT) or predisposition of HIV-positive patients to vitamin B12 deficiency.[1213] This is in line with the known side effect of AZT, as its use is associated with myelosuppression, pure red cell aplasia, and macrocytosis, especially among females.[81718] The combination of myelosuppression and pure red cell aplasia may be responsible for the low hematocrit while the macrocytosis can account for the high MCV among HIV-positive pregnant women on AZT containing ARV regimen.
Low total leukocyte count and its differentials were reported by many studies among HIV-positive women in comparison to their HIV-negative counterpart.[192021] Furthermore, low leukocyte and its differentials were similarly reported by Mwinga et al. among African HIV-positive pregnant women.[13] Myelosuppression from HIV itself, cytokine response or ARV drugs could be responsible for the low WBC and its differential among HIV-positive pregnant. Alternatively, low WBC and its differential can be a consequence of depletion of CD4+ T-cells, neutropenia or combination of these as both can occur from diverse mechanisms such as decreased serum level of granulocyte-colony stimulating factor, cell bound immunoglobulin, increased apoptosis, cytopathic effect of the virus and loss of immature precursors among other mechanisms. However, finding of low WBC and lymphocyte among HIV-positive women with CD4 <350 μ/L is additional evidence to suggest CD4 T-cell depletion as a cause of leukopenia in HIV patient, and it could be related to the contribution of CD4+ T-cells to the lymphocytes and total WBC.
While some studies reported the statistically significant difference in terms of platelet count between HIV-positive and negative pregnant women.[1213] This study did not find any statistically significant difference in platelet count of the two groups, and this could result from the effect of ARV or some geographic or ethnic differences.
Although, all the pregnant women that participated in the study were on hematenics and had intermittent prevention therapy for malaria as at when due, participants in this study were not screened for malaria and serum/red cell levels of the hematenics (folate, B12, and iron) were not assayed. All these could potentially affect some of the hematological parameters analyzed in the study like hematocrit and WBC counts. Therefore, these are some of the limitations of the study.
Conclusions
There is a remarkable difference between the hematological parameters between HIV positive and HIV-negative pregnant women in this environment. Pregnant women with HIV tend to have low hematocrit and high ESR than HIV-negative women. Furthermore, even among HIV-positive pregnant women some differences in hematological parameters are noticeable on the basis of the CD4 count and type ARV regimen.
We recommended that this should be taken into consideration while interpreting results of these parameters and further research is needed to fill in the gaps left by this study such as the role malaria and serum/red cell levels of hematenics on the hematological parameters of HIV-positive pregnant women on ARV therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Nigerian National HIV/AIDS Prevention Plan (2014-2015). Abuja, Nigeria: Federal Ministry of Health; 2013.
- Integrated National Guidelines for HIV Prevention, Treatment and Care. National AIDS/STIs ontrol Programme. Abuja, Nigeria: Federal Ministry of Health; 2014.
- Physiological changes in hematological parameters during pregnancy. Indian J Haematol Blood Transfus. 2012;28:144-6.
- [Google Scholar]
- Is there any difference in haematological parameters in pregnant and non-pregnant women? Natl J Community Med. 2015;6:429-32.
- [Google Scholar]
- Profile of hematological abnormalities of Indian HIV infected individuals. BMC Blood Disord. 2009;9:5.
- [Google Scholar]
- Some haematological parameters in human immunodeficiency virus (HIV) infected Africans: The Nigerian perspective. Niger J Med. 2005;14:33-8.
- [Google Scholar]
- Adverse events in HIV infected children on antiretroviral therapy at a teaching hospital in Lagos, Nigeria: A retrospective study. Adv Pharmacoepidemiol Drug Saf. 2012;1:117.
- [Google Scholar]
- Routine versus clinically driven laboratory monitoring and first-line antiretroviral therapy strategies in African children with HIV (ARROW): A 5-year open-label randomised factorial trial. Lancet. 2013;381:1391-403.
- [Google Scholar]
- Changes in haematological indices in normal pregnancy. Physiol J 2013 Doi: 101155/2013/283814
- [Google Scholar]
- Haematological abnormalities in treatment naïve HIV patients. Infect Dis Res Treat. 2010;3:45-9.
- [Google Scholar]
- HIV-associated anaemia before and after initiation of antiretroviral therapy at Art Centre of Minilik II Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2012;50:13-21.
- [Google Scholar]
- The impact of maternal HIV infection on cord blood lymphocyte subsets and cytokine profile in exposed non-infected newborns. BMC Infect Dis. 2011;11:38.
- [Google Scholar]
- Selected haematological and biochemical measurements in African HIV infected and uninfected pregnant women and their infants: The HIV prevention trial network 024 protocol. BMC Paediatr. 2009;9:49.
- [Google Scholar]
- Assessment of haematological parameters in HIV-infected and uninfected Rwandan women: A cross-sectional study. BMJ Open. 2012;2:pii: E001600.
- [Google Scholar]
- Anemia in HIV infection: Clinical impact and evidence-based management strategies. Clin Infect Dis. 2004;38:1454-63.
- [Google Scholar]
- Prognostic importance of anaemia in HIV type-1-infected patients starting antiretroviral therapy: Collaborative analysis of prospective cohort studies. Antivir Ther. 2008;13:959-67.
- [Google Scholar]
- Adverse effects of antiretroviral therapy in HIV-1 infected children. J Trop Pediatr. 2006;52:244-8.
- [Google Scholar]
- Pathogenesis of HIV infection: Total CD4+ T-cell pool, immune activation, and inflammation. Top HIV Med. 2010;18:2-6.
- [Google Scholar]
- Comparisons of anemia, thrombocytopenia, and neutropenia at initiation of HIV antiretroviral therapy in Africa, Asia, and the Americas. Int J Infect Dis. 2010;14:e1088-92.
- [Google Scholar]





