Translate this page into:
Histopathological array of lesions in nephrectomies with emphasis on immunohistochemical expression of carbonic anhydrase IX in renal cell carcinoma – A 5-year experience in South India
*Corresponding author: Ramya Katta, Department of Pathology, Guntur Medical College, Guntur, Andhra Pradesh, India. drkattaramya@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Medidi R, Gaddam S, Duvvuri P, Katta R. Histopathological array of lesions in nephrectomies with emphasis on immunohistochemical expression of carbonic anhydrase IX in renal cell carcinoma – A 5-year experience in South India. J Lab Physicians. 2024;16:466-74. doi: 10.25259/JLP_19_2024
Abstract
Objectives:
The present study aimed to assess various pathological entities encountered in nephrectomy specimens received in our tertiary care teaching hospital in South India to assess the immunohistochemical expression of carbonic anhydrase IX (CA IX) in various subtypes of renal cell carcinomas (RCCs), and to evaluate its usefulness as a diagnostic and prognostic marker in RCCs.
Materials and Methods:
The present study was a 5-year retrospective study performed in a teaching hospital. All nephrectomy specimens were routinely fixed, grossed, processed, and sectioned for histopathological evaluation. Immunohistochemistry for CA IX expression was performed manually in all cases diagnosed as RCCs.
Statistical analysis:
All data were tabulated in Microsoft Excel 2020, and means and standard deviations were calculated using the same.
Results:
A total of 102 patients were included in the study, with the most common age group being the fifth decade of life and female preponderance. CA IX expression was observed in 100% of clear cell carcinomas, and staining intensity varied with the nuclear grade of the tumor.
Conclusions:
Non-neoplastic lesions were the most common cause of nephrectomy in our study region. Clear cell RCC is associated with the highest expression of CA IX and seems to be associated with lower nuclear grade and lower T stage; hence, it may serve as a prognostic marker for such cases.
Keywords
Pyelonephritis
Hypoxia-inducible factor
Clear cell renal cell carcinoma
Chromophobe renal cell carcinoma
Nephroblastoma
INTRODUCTION
Kidney disease is one of the leading causes of morbidity and mortality across the world.[1] It accounts for 1–3% of all malignant viscera.[2] Renal function tests play a pivotal role in the detection of kidney function and help render appropriate diagnosis and treatment. Kidney diseases include a large array of congenital, traumatic, inflammatory, infectious, vascular, obstructive, and neoplastic (benign and malignant) conditions. Any of these, leading to advanced kidney damage or end-stage renal disease, prompts surgical removal of the kidney as the only treatment option. Nephrectomies are of various types, including partial, simple, and radical, and are performed according to the disease course.
Indications for nephrectomy show geographic variations based on the epidemiology of kidney disease. In India, nonneoplastic conditions resulting in non-functioning kidneys remain the most common indication for nephrectomies, as opposed to developed countries, where renal malignancies are more common.[3]
Of the kidney tumors, malignant tumors outnumber benign tumors significantly, with renal cell carcinoma (RCC) and Wilm’s tumor being the most common type.[4] Renal carcinogenesis, as in other types of cancer, involves the interplay of many molecular and signaling pathways.[4] The primary risk factors include tobacco use and alcoholism. Carbonic anhydrase IX (CA IX) is a commonly used biomarker for clear cell carcinoma.[4] It is a transmembrane metalloprotease that is expressed physiologically in the gastrointestinal tract. Its activity is controlled by hypoxiainducible factor-1 (HIF1). Its role in tumorigenesis has been proven in many cancers, and it is known to be involved in neo-angiogenesis, cell migration, and metastasis. However, its overall sensitivity and specificity in diagnosing various subtypes and its role in the prognostication of clear cell RCCs are still nebulous.[4]
The objectives of the present study were to assess various pathological entities encountered in nephrectomy specimens received in our hospital, to assess the immunohistochemical expression of CA IX in various subtypes of RCCs, and to evaluate its usefulness as a diagnostic and prognostic marker in RCCs.
MATERIALS AND METHODS
Study design and duration: A tertiary care teaching hospital-based retrospective descriptive study was conducted over 5 years (January 2019–December 2023) after obtaining Institutional Ethics clearance (GMC/IEC/108/2023, dated June 21, 2023).
Inclusion criteria: All nephrectomy specimens (partial, simple, and radical) that were received during the study period were included in the study.
Exclusion criteria: Patients with a previous history of surgery, re-exploration surgery specimens, and prior neoadjuvant therapy were excluded.
All specimens were routinely fixed in 10% buffered formalin, processed in an automatic tissue processor (Leica TP 1020), and stained with hematoxylin and eosin (H and E). Two experienced pathologists with clinical correlation reviewed all cases. All malignant tumors were classified according to the World Health Organization’s classification of urinary and male genital tumors in 2022.[5]
Representative blocks of all cases that were diagnosed as RCC subtypes were retrieved from the departmental archives and manual immunohistochemistry was performed prospectively using CA IX antibody (rabbit monoclonal antibody clone EP161, Pathnsitu Biotechnologies) according to the manufacturers’ recommendations and protocols. Antigen retrieval was performed in a microwave oven. Semi-quantitative assessment of the immunohistochemical expression of CA IX was performed using a scoring system that has been previously described.[6-8] Cases that showed a high-intensity box-like pattern of membranous staining in >85% of the tumor cells were considered high-expressing tumors, those with ≤85% membranous staining on the tumor cells were considered low-expressing tumors, and cases with no membranous staining were considered to be negative for CAIX expression.[6-8]
Statistical analysis
Data analysis, tabulation, and statistical processing were performed using Microsoft Excel 2020. Frequencies and percentages were calculated for discrete data, and mean and standard deviations were calculated for continuous data.
RESULTS
After considering all inclusion and exclusion criteria, 102 specimens were included in the present study. As depicted in Table 1, age of the patients ranged between 1 month of age and 75 years of age, with the most common age group being the 5th decade of life and with a minor female preponderance (male: female ratio = 0.85:1). In the present study, 49.1% of patients underwent nephrectomy due to chronic kidney disease/end-stage renal disease resulting in uremia, and the most common surgical procedure was simple nephrectomy in 67.8% of cases [Table 2]. The histopathological diagnoses encountered in the present study are listed in Table 3.
| Age range (years) | Number of males (%) | Number of females (%) | Number of non-neoplastic lesions (%) | Number of neoplastic lesion (%) | Total (%) |
|---|---|---|---|---|---|
| <1 | 3 (2.9) | 2 (2.0) | 2 (1.9) | 3 (2.9) | 5 (4.9) |
| 1–10 | 3 (2.9) | 3 (2.9) | 4 (3.9) | 2 (2.0) | 6 (5.8) |
| 11–20 | 3 (2.9) | 1 (0.9) | 4 (3.9) | 0 (0) | 4 (3.9) |
| 21–30 | 5 (4.9) | 9 (8.8) | 11 (10.7) | 3 (2.9) | 14 (13.8) |
| 31–40 | 10 (9.8) | 7 (6.9) | 13 (12.7) | 4 (3.9) | 17 (16.7) |
| 41–50 | 10 (9.8) | 19 (18.6) | 21 (11.7) | 8 (7.8) | 29 (28.5) |
| 51–60 | 9 (8.8) | 11 (10.7) | 13 (12.7) | 7 (6.9) | 20 (19.6) |
| 61–70 | 4 (3.9) | 0 (0) | 0 (0) | 4 (3.9) | 4 (3.9) |
| 71–80 | 0 (0) | 3 (2.9) | 2 (1.9) | 1 (1.0) | 3 (2.9) |
| Total | 47 (46.1) | 55 (53.9) | 70 (68.6) | 32 (31.4) | 102 (100) |
| Type of nephrectomy | Number of cases (%) |
|---|---|
| Partial | 12 (11.8) |
| Simple | 69 (67.6) |
| Radical | 21 (20.6) |
| Clinical features | |
| Uremia | 50 (49.1) |
| Mass per abdomen | 8 (7.8) |
| Hematuria | 14 (13.7) |
| Flank pain | 12 (11.8) |
| Burning micturition | 18 (17.6) |
| S. No. | Broad diagnosis | Subgroup | Histopathological diagnosis | n(%) |
|---|---|---|---|---|
| 1. | Non-neoplastic lesions | Interstitial diseases | Chronic pyelonephritis | 51 (50) |
| Xanthogranulomatous pyelonephritis | 4 (3.9) | |||
| Granulomatous pyelonephritis | 2 (2.0) | |||
| Cystic diseases | Simple cysts | 1 (0.9) | ||
| Obstructive diseases | Chronic pyelonephritis with hydronephrosis changes | 9 (8.8) | ||
| Chronic pyelonephritis with renal calculi | 3 (2.9) | |||
| 2. | Neoplastic lesions | Renal cell tumors | Clear cell renal cell carcinoma | 12 (11.8) |
| Papillary renal cell carcinoma | 2 (2.0) | |||
| Chromophobe renal cell carcinoma | 3 (2.9) | |||
| Metanephric tumors | Metanephric adenoma | 1 (0.9) | ||
| Nephroblastic tumors | Nephroblastoma | 3 (2.9) | ||
| Mesenchymal tumors | Congenital mesoblastic nephroma | 2 (2.0) | ||
| Leiomyosarcoma | 1 (0.9) | |||
| Angiomyolipoma | 2 (2.0) | |||
| Urothelial carcinoma | 2 (2.0) | |||
| Others | Adenomatoid tumor | 1 (0.9) | ||
| Pheochromocytoma | 1 (0.9) | |||
| Metastatic tumors | Metastatic breast carcinoma | 2 (2.0) |
In the present study, non-neoplastic lesions exceeded neoplastic lesions (68.6% and 31.4%, respectively). Among the 70 cases of non-neoplastic lesions, interstitial kidney diseases (57 cases) were the most common lesions, followed by obstructive diseases (12 cases). The most common non-neoplastic lesion in the present study was chronic pyelonephritis (CPN) and most cases presented between 3rd and 5th decades of life [Figure 1a]. In the present study, a total of four cases of xanthogranulomatous pyelonephritis (XPN) and two cases of granulomatous pyelonephritis, probably of tubercular origin, were diagnosed [Figure 1b and c]. All four XPN were diagnosed in female patients of age group 41– 60 years, and both granulomatous pyelonephritis occurred in males aged 42 and 48 years, respectively.
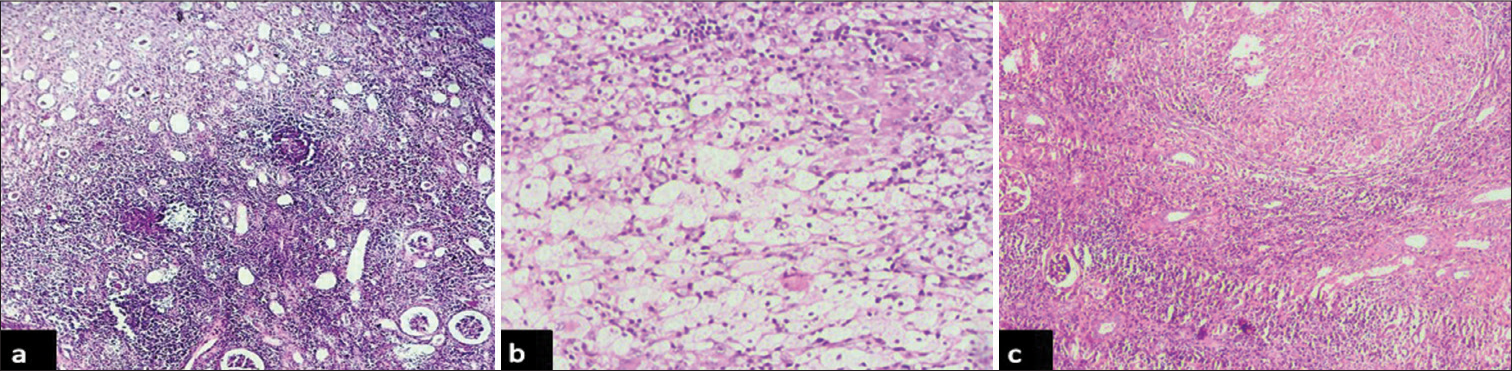
- Photomicrographs of non – neoplastic lesions of kidney: (a) showing thyroidization of renal tubules, sclerotic blood vessels and interstitial inflammation- features consistent with Chronic pyelonephritis (hematoxylin and eosin, x4 objective lens), (b): showing structure of kidney infiltrated by xanthoma cells and chronic inflammatory infiltrate- features consistent with xanthogranulomatous pyelonephritis (hematoxylin and eosin, x40 objective lens), and (c) showing structure of kidney infiltrated by caseating granulomas with giant cells- features consistent with tuberculous pyelonephritis (hematoxylin and eosin, x4 objective lens).
Of the neoplastic lesions encountered in the present study, malignant tumors constituted 84.3% of neoplastic lesions (27 of 32 cases), including 17 cases of RCCs, three cases of nephroblastoma, two cases of congenital mesoblastic nephroma (low malignant potential), two cases of urothelial carcinomas, one case of leiomyosarcoma, and two cases of metastatic tumors, from primary breast malignancy.
The mean age at presentation for RCC in the present study was 51.7 ± 12.5 years (age range, 29–75 years), with a male preponderance (M: F ratio—12:5). Most tumors occurred on the left side (left: right ratio—12:5) and in the lower pole (lower: mid: upper pole—8:6:3). Microscopically, clear cell carcinoma with nuclear grade of 1 was the most common type, followed by chromophobe and papillary types.
In the current cohort, three cases of nephroblastoma were diagnosed in children aged 9 months, 10 months, and 3 years. All the patients presented with a palpable abdominal mass. All three cases were unilateral and unifocal and showed blastemal, epithelial, and stromal components on histological evaluation [Figure 2a and b]. Only one case showed evidence of anaplasia. In the current study, we encountered two cases of congenital mesoblastic nephroma occurring in 1-month and 2-month-old infants. Histologically, both the cases included in the present cohort showed sheets and fascicles of plump-looking cells with vesicular nuclei and a moderate amount of cytoplasm with foci showing islands of cartilage and entrapped renal tubules (mixed variant) [Figure 2c and d].
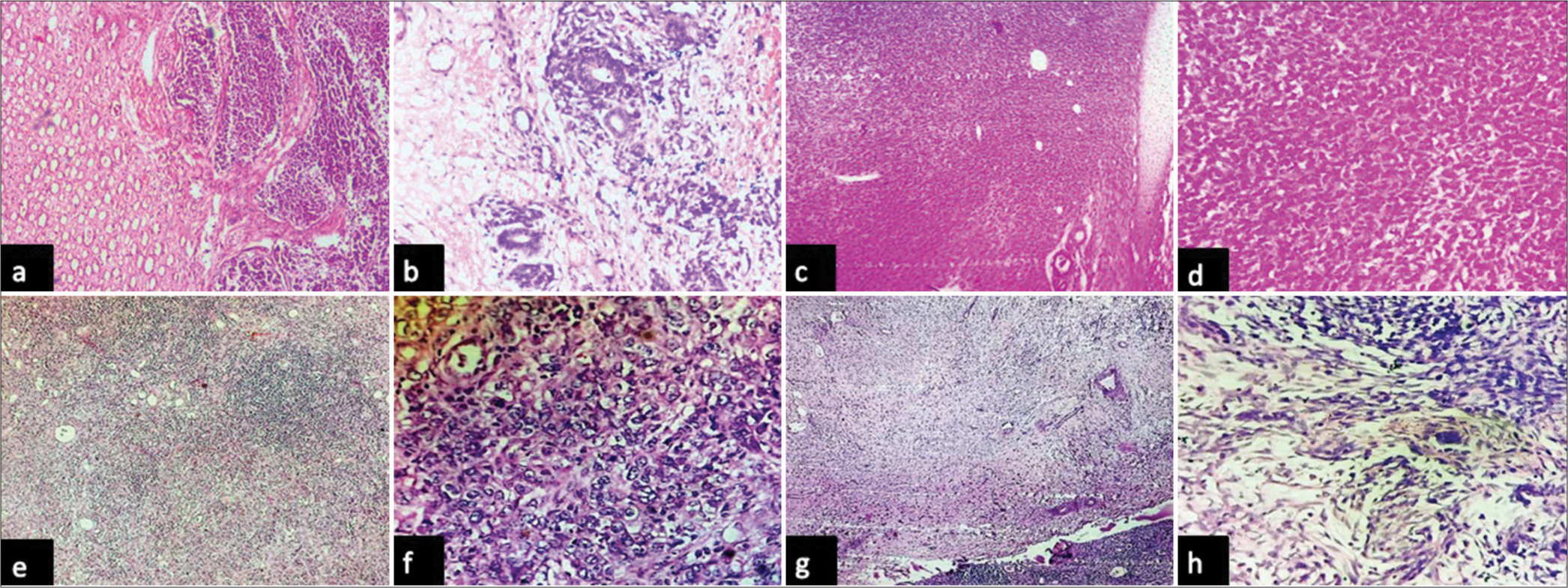
- Photomicrographs: (a and b) Wilms tumor composed of blastemal, epithelial and mesenchymal component with focal anaplasia (hematoxylin and eosin, x4 and x40 objective lens respectively), (c and d) congenital mesoblastic nephroma composed of plump cells arranged in sheets with islands of hyaline cartilage (hematoxylin and eosin, x4 and x40 objective lens respectively), (e and f) urothelial carcinoma of the renal pelvis composed of pleomorphic cells arranged in sheets seen infiltrating renal parenchyma with prominent interstitial inflammation (hematoxylin and eosin, x4 and x40 objective lens respectively), and (g and h) showing structure of kidney with leiomyosarcoma composed of pleomorphic spindle shaped cells arranged in fascicles showing focal hyalinization (hematoxylin and eosin, x4 and x40 objective lens respectively).
The two cases of urothelial carcinoma encountered during the present study period were located in the renal pelvis and ureter, and hence, radical nephrectomy was performed. Both tumors occurred in patients aged >50 years and were of a high-grade nature with renal parenchymal infiltration [Figure 2e and f]. A single case of primary leiomyosarcoma of the kidney was included in the present study, which occurred in a 48-year-old female patient who underwent radical nephrectomy [Figures 2g and h].
In the present study, two cases of secondary kidney tumors were identified. Both cases were females aged 46 and 57 years who had undergone mastectomy for breast malignancy 3 and 4 years ago and presented with renal masses. Histological examination showed evidence of tumor deposits from the primary breast in the renal sinus and renal pelvis, with CPN changes in the adjacent kidney [Figure 3a-c]. Immunohistochemical analysis of the estrogen receptors confirmed the diagnosis.
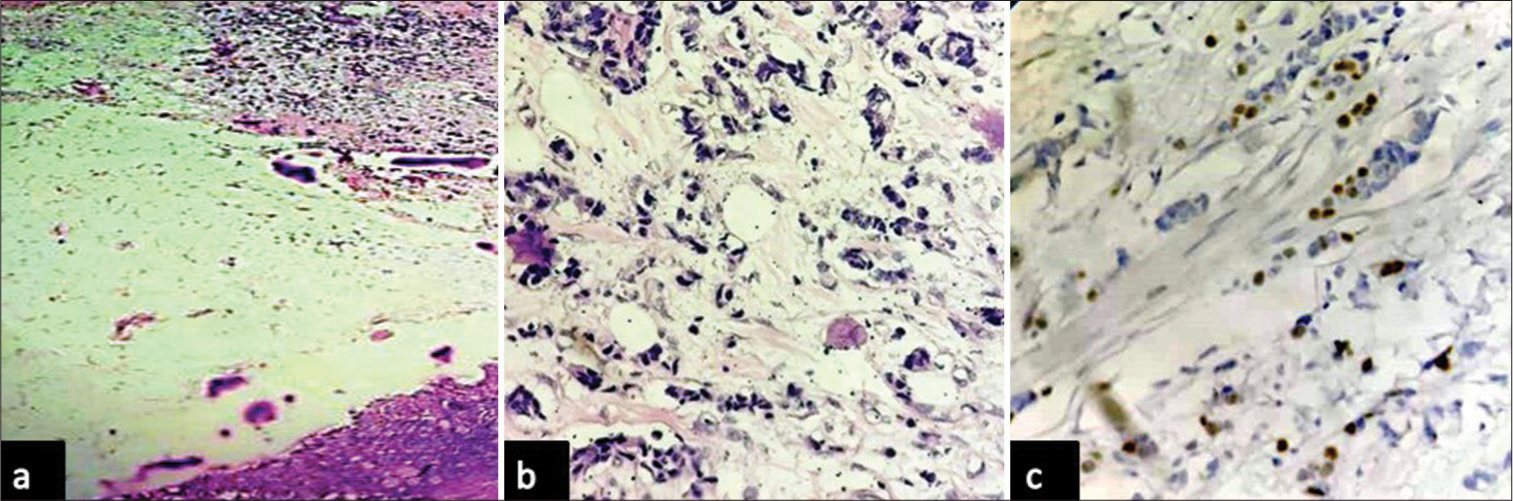
- Photomicrographs of metastatic breast carcinoma: (a and b) showing renal parenchyma with metastatic deposit from breast carcinoma (hematoxylin and eosin, x4 and x40 objective lens respectively), and (c) showing tumor cells with nuclear positivity for estrogen receptor (diaminobenzidine, x40 objective lens).
Benign tumors encountered in the present study include two cases of angiomyolipoma, one case each of metanephric adenoma, adenomatoid tumor, and pheochromocytoma. We reported two cases of angiomyolipoma in two female patients aged 39 and 40 years, respectively [Figure 4a and b]. Both cases were diagnosed preoperatively, and a simple nephrectomy was performed. A single case of metanephric adenoma was reported in a 48-year-old male who presented with hematuria and a mass in the left kidney [Figure 4c and d]. A partial nephrectomy was performed. A single case of adenomatoid tumor was reported in a 50-year-old female who presented with dull, aching pain on the right flank region. A computed tomography scan revealed a cystic neoplasm in the upper pole of the right kidney, which was provisionally diagnosed as RCC. However, histopathology revealed a well-circumscribed tumor composed of bland-looking cuboidal to epithelioid cells arranged in tubular and cord-like patterns and was diagnosed as an adenomatoid tumor [Figure 4e and f]. In the present study, one case of pheochromocytoma arising near the pelvis of the kidney was included, which occurred in a 30-year-old female who presented with an enhancing intra-renal mass near the midpole of the right kidney [Figures 4g and h].
- Photomicrographs: (a and b): of angiomyolipoma with myoid spindle cells, mature adipose tissue and blood vessels (hematoxylin and eosin, x4 and x10 objective lens respectively), (c and d): of metanephric adenoma composed of tumor cells arranged in tightly packed tubules and pseudopapillary patterns (hematoxylin and eosin, x10 and x40 objective lens respectively), (e and f): of adenomatoid tumor composed of tumor cells arranged in tubules, cords and focal angiomatoid patterns (hematoxylin and eosin, x4 and x40 objective lens respectively), and (g and h): of pheochromocytoma composed of tumor cells arranged in nesting patterns with adjacent normal looking renal tubules (hematoxylin and eosin, x4 and x40 objective lens respectively).
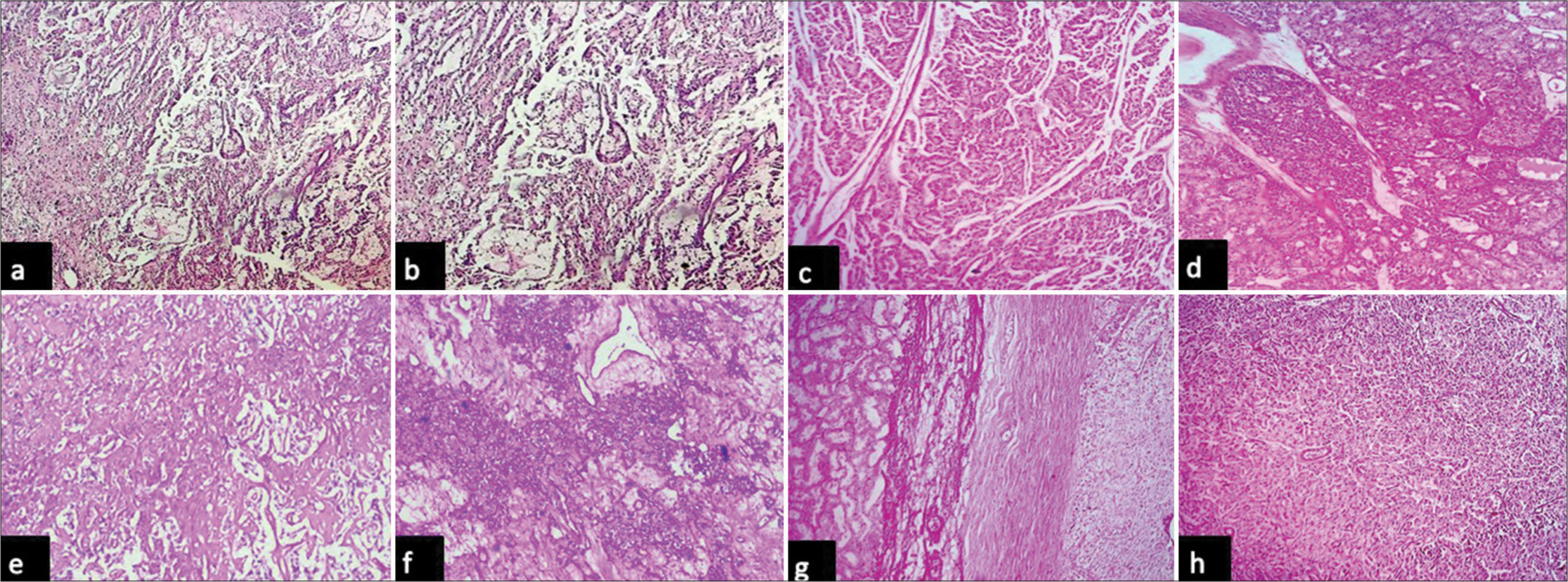
Immunohistochemical analysis of CA IX was performed for all malignant renal epithelial cell tumors included in the present study. CA IX expression was observed in 100% of clear cell carcinomas, and none of the papillary or chromophobe RCCs showed expression in the present study [Table 4]. When clinicopathological parameters of clear cell carcinoma were compared, it was found that high expression of CA IX (>85% of cells with high intensity and box-like pattern) was seen in cases showing low nuclear grade and lower T stage while other features such as age, gender, focality, presence of necrosis, and laterality did not show such association [Table 5, Figure 5a and b]. Cases with higher nuclear grade showed low and focal expression of CA IX (<85% of cells, lower intensity) [Figure 5c-f]. All the cases of chromophobe cell renal cell carcinoma were negative for CAIX [Figure 5g and h]. However, due to the small sample size, statistical analysis could not be performed.
| Histopathological type | Total number of cases | Number of cases showing immunohistochemical CAIX expression (%) |
|---|---|---|
| Clear cell renal cell carcinoma | 12 | 12 (100) |
| Chromophobe renal cell carcinoma | 3 | 0 (0) |
| Papillary renal cell carcinoma | 2 | 0 (0) |
CA IX: Carbonic anhydrase IX
| Clinicopathological parameter | Description | n(%) | CA IX expression in tumor cells | |
|---|---|---|---|---|
| High (%) | Low (%) | |||
| Age | <50 years | 5 (41.6) | 4 (33.3) | 1 (8.3) |
| >50 years | 7 (58.4) | 5 (41.6) | 2 (16.7) | |
| Gender | Males | 10 (83.3) | 8 (66.7) | 2 (16.7) |
| Females | 2 (16.7) | 1 (8.3) | 1 (8.3) | |
| Laterality | Right | 2 (16.7) | 1 (8.3) | 1 (8.3) |
| Left | 10 (83.3) | 8 (66.7) | 2 (16.7) | |
| Focality | Unifocal | 9 (75) | 6 (50) | 3 (25) |
| Multifocal | 3 (25) | 3 (25) | 0 (0) | |
| Nuclear grade (ISUP grading) | Grade 1 | 8 (66.7) | 8 (66.7) | 0 (0) |
| Grade 2 | 3 (25) | 1 (8.3) | 2 (16.7) | |
| Grade 3 | 1 (8.3) | 0 (0) | 1 (8.3) | |
| Grade 4 | 0 (0) | 0 (0) | 0 (0) | |
| Necrosis | Present | 3 (25) | 2 (16.7) | 1 (8.3) |
| Absent | 9 (75) | 7 (58.4) | 2 (16.7) | |
| T stage | T1 | 7 (58.4) | 6 (50) | 1 (8.3) |
| T2 | 1 (8.3) | 1 (8.3) | 0 (0) | |
| T3 | 4 (33.3) | 2 (16.7) | 2 (16.7) | |
CA IX: Carbonic anhydrase IX, ISUP: International society of urological pathologists
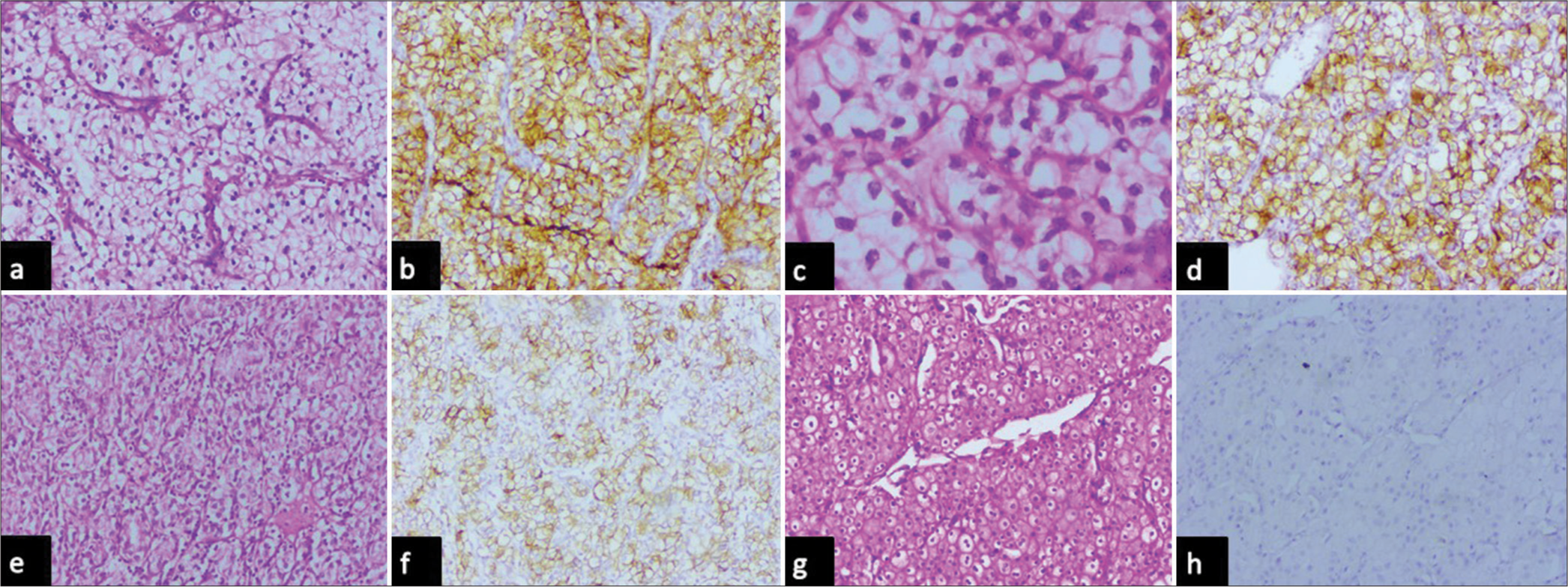
- Photomicrographs of (a and b) clear cell renal cell carcinoma with nuclear grade 1 showing box like intense membrane positivity (high) for carbonic anhydrase IX (hematoxylin and eosin, x10 objective lens and diaminobenzidine, x10 objective lens respectively), (c and d) clear cell renal cell carcinoma with nuclear grade 2 membrane positivity in less than 85% of tumor cells for carbonic anhydrase IX (hematoxylin and eosin, x40 objective lens and diaminobenzidine, x10 objective lens respectively), (e and f) clear cell renal cell carcinoma with nuclear grade 3 showing less than 85% of tumor cells for carbonic anhydrase IX (hematoxylin and eosin, x10 objective lens and diaminobenzidine, x10 objective lens respectively), and (g and h) chromophobe cell renal cell carcinoma showing no expression of Carbonic anhydrase IX (hematoxylin and eosin, x10 objective lens and diaminobenzidine, x10 objective lens respectively).
DISCUSSION
Nephrectomy is a common surgical procedure for a wide variety of renal diseases, including benign and malignant diseases. A literature review of nephrectomies ascertains that the cause of nephrectomy is highly dependent on socioeconomic status, access to health care, dietary habits, and the geographic terrain of a particular area.[9,10] Most studies conducted in Asia reported a higher incidence of non-neoplastic lesions[11-16] in nephrectomy specimens, whereas the opposite was true in developed nations.[17,18] The most common clinical feature in this study was uremia, followed by burning micturition; most malignant tumors presented with hematuria. Our findings are in agreement with other studies.[3,13,15]
CPN constituted the most common non-neoplastic lesion in the present study. The most common forms of CPN include reflux nephropathy and chronic obstructive pyelonephritis.[18] Loss of corticomedullary differentiation, presence of irregular scars, and blunted and dilated renal calyces were the most evident gross findings in most CPNs in the present study. XPN is an unusual and severe form of chronic bacterial infection that most commonly results from the obstruction of urinary flow.[19] XPN was diagnosed when extensive renal parenchymal tissue damage and granulomatous inflammation were observed histologically. All four cases were diagnosed in female patients of age group 41–60 years. Our findings are in correlation with that of Aiman et al.[3] In the current series, two cases of granulomatous pyelonephritis showed granulomas composed of central caseating necrosis surrounded by epithelioid macrophages. However, tubercular bacilli could not be identified.
As in other studies,[12,15] the majority of malignant cases in the present cohort were RCCs. RCCs constitute a pathologically heterogeneous group of malignancies with overlapping or distinct architectural, cytological, and morphological features. Correct subtyping helps with prognostication and individualized treatment.[4] In most cases, routine H and E-stained sections serve an accurate diagnosis. However, immunohistochemistry will aid in diagnosis in certain cases where viable tumor tissue is scant. Small core biopsies are provided in identifying metastatic deposits.[4] Popat et al.[12] and Rafique [15], in their studies, recognized that the majority of the RCCs occurred in the upper pole, while the present series reported the lower pole to be the most common site. Clear cell carcinoma was the most common type in the present study. This finding is in correlation with other studies.[12,15] However, most cases of classical clear cell carcinoma reported in the present cohort had a nuclear grade of 1 (eight cases). This finding is in contrast to many studies[2,12,15], which observed that nuclear grade 2 and nuclear grade 3 were more common.
Nephroblastoma or Wilms tumor is an embryonal tumor derived from nephrogenic blastemal cells and is the fourth most common pediatric malignancy the world over.[20] Our findings of presentation as a palpable abdominal mass and the presence of all three histological components are consistent with the observations of Kinoshita et al.[20]
Congenital mesoblastic nephroma is the most common congenital renal neoplasm, accounting for 2% of all pediatric renal tumors. These tumors occur most commonly in early infancy and are of fibroblastic origin with low malignant potential.[21] It is important to differentiate these tumors from nephroblastoma as treatment options and their prognosis varies.[21]
Urothelial carcinoma is the fourth most common malignancy worldwide.[22] 90–95% of them occur in the urinary bladder. Less common sites include the renal pelvis, ureters, and urethra.[22] The findings of the present study are consistent with those reported by Tian et al.[23]
Andrea et al.[24] in their study found that most leiomyosarcomas of kidney occurred more commonly in females with a mean age of 58 years which is consistent with the findings of the present study.
The involvement of the kidney in metastatic tumors has been commonly reported in autopsy studies of patients with malignancies. However, with the use of better imaging modalities, metastatic tumors of the kidneys are being identified more often.[25] Saeed and Osunkoya[25] in their study found that the lung was the most common primary site while the present study reported the breast to be the most common primary site.
Angiomyolipoma is a benign mesenchymal tumor composed of varying proportions of adipose tissue, smooth muscle fibers, and thickened blood vessels, arising either sporadically or in association with tuberous sclerosis.[26] The two cases included in the present study occurred sporadically, and bulky fat content was useful for pre-operative radiological diagnosis.
Metanephric adenoma is a rare renal epithelial tumor making up for about 0.2% of all renal neoplasms.[27] Adenomatoid tumors of the genitourinary tract are infrequent benign neoplasms of mesothelial origin.[28] Diagnosing benign tumors of the kidney on radiological imaging is very challenging as most cases mimic RCCs.[28,29] Intraoperative microscopic examination (frozen section) can be very useful in such cases as it can guide treatment.[28,29] The single case of intrarenal pheochromocytoma included in the present study probably may have arisen from intrarenal adrenal ectopic nests.[29]
In most malignant tumors, excessive growth and proliferation result in hypoxic microenvironment. This niche results in tumor cell adaptation by altering many molecular signaling pathways, which help in neoangiogenesis, invasion, and metastasis which finally incur the tumor cell a property of resistance to therapy. CA IX plays a key role in this. It is a zinc-containing transmembrane metalloprotease that is involved in acid-base balance, cell adhesion, and cellular proliferation. Its expression is controlled by Von Hippel–Lindau protein (VHL) and downstream HIF-1α. Since 95% of clear cell RCCs have a constitutional loss of the VHL gene, clear cell carcinomas have the highest expression of CA IX. However, its expression in other renal epithelial tumors is variable, as per the literature. In the present study, which includes a small sample size, it was found that CA IX was 100% specific for clear cell carcinomas with nil expression in chromophobe and papillary subtypes. Baniak et al.[4] in their study observed that CA IX expression was not entirely specific for clear cell RCCs and that it could be expressed in clear cell tubulopapillary variant of RCC, xp11.2 RCC, and fumarate hydratase-deficient RCC. All be it, our finding of high expression of CA IX in clear cell carcinoma with lower nuclear grade and lower T stage is consistent with many studies and hence may be used as a prognostic marker.[30,31]
Limitations
The present study, being a retrospective analysis and small sample size for immunohistochemical analysis could have a possibility of bias.
CONCLUSIONS
The present study provides insight into various histological lesions that were encountered in nephrectomies in our region with the comparison of worldwide data. Clear cell RCC is associated with the highest expression of CA IX and seems to be associated with lower nuclear grade and hence may serve as a prognostic marker for such cases. However, for substantiation, a study on a larger population is crucial.
Author contributions
RM, SG, P.SRD, RK: Data acquisition, manuscript preparation, editing and final proofing.
Ethical approval
The research/study was approved by the Institutional Ethics Committee, Guntur Medical College, Guntur, number GMC/IEC/108/2023, dated 21st June 2023.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
- [CrossRef] [PubMed] [Google Scholar]
- Study of pattern of histopathological lesions in nephrectomy cases in Southern Rajasthan. J Clin Diagn Res. 2020;14:EC05-8.
- [CrossRef] [Google Scholar]
- Histopathological spectrum of lesions in nephrectomy specimens: A five year experience in a tertiary care hospital. J Sci Soc. 2013;40:148-54.
- [CrossRef] [Google Scholar]
- Carbonic anhydrase IX (CA9) expression in multiple renal epithelial tumour subtypes. Histopathology. 2020;77:659-66.
- [CrossRef] [PubMed] [Google Scholar]
- Urinary and male genital tumours Vol 5. Lyon, France: International Agency for Research on Cancer; 2022. p. :128.
- [Google Scholar]
- Carbonic anhydrase IX expression in renal neoplasms: Correlation with tumor type and grade. Am J Clin Pathol. 2010;134:873-9.
- [CrossRef] [PubMed] [Google Scholar]
- Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9:802-11.
- [Google Scholar]
- Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11:3714-21.
- [CrossRef] [PubMed] [Google Scholar]
- Nutritional management of kidney stones (nephrolithiasis) Clin Nutr Res. 2015;4:137-52.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathological spectrum of nephrectomy specimen in a tertiary care center: With an emphasis on chronic pyelonephritis. Ann Pathol Lab Med. 2017;4:573-8.
- [CrossRef] [Google Scholar]
- Analysis of 88 Nephrectomies in a rural tertiary care center of India. Saudi J Kidney Dis Transpl. 2002;23:409-13.
- [Google Scholar]
- A study on culprit factors, ultimately demanding nephrectomy. Internet J Urol. 2009;7:1-8.
- [CrossRef] [Google Scholar]
- Prevalence of pathological lesions in 161 nephrectomies; An experience from a teaching hospital in urban industrial area of Maharashtra. Indian J Med Special. 2020;11:21-7.
- [CrossRef] [Google Scholar]
- Nephrectomy in adults: ASIR Hospital experience. Sandi J Kidney Dis Transplant. 1977;8:423-7.
- [Google Scholar]
- Nephrectomy: Indications, complications and mortality in 154 consecutive patients. J Pak Med Assoc. 2007;57:308-11.
- [Google Scholar]
- Nephrectomy indications, complications and post-operative mortality in 646 consecutive patients. Eur Urol. 2000;37:58-64.
- [CrossRef] [PubMed] [Google Scholar]
- The kidney In: Kumar V, Abbas AK, Aster JC, eds. Robbins pathologic basis of disease (10th ed). Philadelphia, PA: Elsevier; 2021. p. :895-952.
- [Google Scholar]
- Xanthogranulomatous pyelonephritis: A systematic review of treatment and mortality in more than 1000 cases. BJU Int. 2023;131:395-407.
- [CrossRef] [PubMed] [Google Scholar]
- The prognostic significance of blastemal predominant histology in initially resected Wilms' tumors: A report from the Study Group for Pediatric Solid Tumors in the Kyushu Area, Japan. J Pediatr Surg. 2012;47:2205-9.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital mesoblastic nephroma fifty years after its recognition: A narrative review. Pediatr Blood Cancer. 2017;64:e26437.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic and therapeutic challenges of rare urogenital cancers: Urothelial carcinoma of the renal pelvis, ureters and urethra. World J Oncol. 2021;12:20-7.
- [CrossRef] [PubMed] [Google Scholar]
- Urothelial carcinoma of the renal pelvis with renal vein and inferior vena cava tumor thrombus: Case series and literature review. Transl Androl Urol. 2021;10:2879-88.
- [CrossRef] [PubMed] [Google Scholar]
- Leiomyosarcoma of the kidney: A clinicopathologic study. Am J Surg Pathol. 2004;28:178-82.
- [CrossRef] [PubMed] [Google Scholar]
- Secondary tumors of the kidney: A comprehensive clinicopathologic analysis. Adv Anat Pathol. 2022;29:241-51.
- [CrossRef] [PubMed] [Google Scholar]
- Update on the diagnosis and management of renal angiomyolipoma. J Urol. 2016;195:834-46.
- [CrossRef] [PubMed] [Google Scholar]
- Metanephric adenoma of the kidney: An unusual diagnostic challenge. Rare Tumors. 2010;2:e38.
- [CrossRef] [PubMed] [Google Scholar]
- Adenomatoid tumor of kidney-a rare histological entity: Case report with review of literature. Urol Nephrol Open Access J. 2015;2:44-5.
- [CrossRef] [Google Scholar]
- Paraganglioma of the renal pelvis: A case report and review of literature. Tumori. 2017;103(Suppl 1):e47-9.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of carbonic anhydrase IX immunohistochemical expression in renal cell carcinoma: A meta-analysis of the literature. PLoS One. 2014;9:e114096.
- [CrossRef] [PubMed] [Google Scholar]
- Carbonic anhydrase IX in renal cell carcinoma, implications for disease management. Int J Mol Sci. 2020;21:7146.
- [CrossRef] [PubMed] [Google Scholar]






