Translate this page into:
Immature Platelet Fraction: Its Clinical Utility in Thrombocytopenia Patients
Address for correspondence: Deepti Joshi, MBBS, MD, Department of Pathology and Laboratory Medicine, All India Institute of Medical Sciences, Bhopal - 462024, Madhya Pradesh, India (e-mail: deepti.patho@aiimsbhopal.edu.in).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
Etiology of thrombocytopenia is multifactorial and its pathogenesis should be distinguished for appropriate management. Newly formed immature platelets are called reticulated platelets (RPs) and can be estimated in peripheral blood using automated hematology analyzers, which express them as immature platelet fraction (IPF). In the present study we intend to assess and establish the clinical utility of IPF in differentiating the two major causes of thrombocytopenia—decreased production and increased destruction of platelets—along with determining its significance in monitoring patients with thrombocytopenia.
Materials and Methods
Sixty-one cases of thrombocytopenia and 101 healthy controls with normal platelet count were included in the study. IPF and all the other usual blood cell parameters were measured using a fully automated hematology analyzer. Based on the pathogenesis of thrombocytopenia, the cases were divided into groups and the difference in IPF value between the groups was evaluated.
Results
The reference range of IPF among healthy controls was estimated to be 0.7 to 5.7%. The mean IPF was significantly higher in patients with increased peripheral destruction of platelets (13.4%) as compared to patients with decreased production of platelets (4.6%). The optimal cutoff value of IPF for differentiating patients with increased peripheral destruction of platelets from patients with decreased production of platelets was 5.95% with a sensitivity of 88% and specificity of 75.9%.
Conclusion
Measurement of IPF is useful for detecting evidence of increased platelet production and helps in the initial evaluation of thrombocytopenia patients. It is a novel diagnostic method which can be used to differentiate patients with thrombocytopenia due to increased destruction of platelets from patients with thrombocytopenia due to bone marrow failure/suppression.
Keywords
thrombocytopenia
immature
platelet
fraction
reticulated
Introduction
A decrease in the number of platelets in peripheral blood, termed as thrombocytopenia, is frequently encountered hematological abnormality and is at times associated with severe bleeding complications. The etiology of thrombocytopenia is multifactorial which makes diagnosis of underlying cause challenging and problematic.[1-3] The pathogenesis of thrombocytopenia which includes either increased peripheral destruction of platelets or decreased production of platelets should be distinguished for appropriate management.[4]
A precise and correct platelet count provides restricted information for the differential diagnosis or bleeding tendency in thrombocytopenia patients, but a quick estimation of platelet production helps in differentiating between thrombocytopenia due to bone marrow failure with increased risk of associated bleeding from thrombocytopenia due to increased platelet consumption where bleeding is less likely.[2,5]
Newly formed immature platelets are more reactive than mature platelets due to high granule content and contain residual RNA derived from megakaryocytes and are called reticulated platelets (RPs).[6] A measure of RP determines the rate of thrombopoiesis in turn helping in the differential diagnosis of thrombocytopenia. A measure of production of RP extends the possibility to establish the etiology of thrombocytopenia, being either increased platelet consumption or bone marrow failure/suppression, without doing a bone marrow examination which is both an invasive and painful procedure for the patient.[7,8]
The quantification of RP can be done using flow cytometers and fluorescent dyes which can bind to the RNA; however, it has limited clinical advantage due to lack of standardization and variation in reference intervals.[9] The estimation of RP in peripheral blood can be done using automated hematology analyzers, which express it as an immature platelet fraction (IPF). IPF measures the immature platelets as a fraction of total number of platelets in peripheral blood.[6] Automated measurement of IPF is reliable and available in daily clinical practice.[10,11]
IPF not only differentiates increased platelet consumption from bone marrow failure/suppression, it also is an early indicator of platelet recovery and can save unnecessary platelet transfusions and the risks associated with it. IPF has a positive correlation with platelet count recovery in patients with thrombocytopenia and can be used to monitor patients with thrombocytopenia.[7,12] In the present study we intend to assess and establish the clinical utility of IPF in diagnosis and monitoring of patients with thrombocytopenia.
Materials and Methods
This was a prospective cross sectional study performed in our hospital where 101 individuals with normal platelet count and other blood cell parameters were registered in the study to establish the normal reference range of IPF in healthy population. These healthy individuals formed the control population in the study. A total of 61 patients with thrombocytopenia were included in the study to assess the clinical utility of IPF in thrombocytopenia patients. These patients formed the case group in the study. The cases and controls were matched for age and gender.
Inclusion Criteria
Cases
Patients with platelet count < 100 × 109/L.
Controls
Apparently healthy individuals with normal blood cell parameters including a normal platelet count ranging from 150 to 450 × 109/L.
Exclusion Criteria
Cases
Patients with pseudothrombocytopenia that is decreased platelet count on analyzer but presence of platelets on peripheral smear.
Patients with severe bleeding which can be fatal.
Patients with known platelet or coagulation disorder.
Controls
Individuals with fever or viral infections.
Individuals with chronic diseases.
Individuals with any thrombotic or hemorrhagic disorders.
The peripheral blood sample from the controls and cases was collected in K2EDTA vials. Informed and written consent was obtained from all the cases and controls. To minimize the variations in the samples due to sample aging the collected samples were analyzed within 2 to 3 hours of collection using Mindray BC-6800.
IPF and all the other usual blood cell parameters including platelet count were measured. The measurement of IPF was done using the flow cytometry technique. It identified the mature and IPFs on basis of their fluorescence intensity and the software stated the IPF as a proportional value of total optical platelet count.
Statistical Analysis
The statistical analysis was done using IBM SPSS 21 statistical software. The controls were grouped according to age and gender and the descriptive analysis was done where mean, median, and standard deviation were calculated for platelet count and IPF. The normal reference range of IPF was established using the data. Depending on the normal reference range the abnormality in the IPF percentage was evaluated in cases with thrombocytopenia.
The cases were grouped according to age, gender, final diagnosis, and pathogenesis of thrombocytopenia. Based on the pathogenesis of thrombocytopenia the cases were divided into two groups: Group 1 with decreased platelet production due to bone marrow failure/suppression and Group 2 with increased peripheral destruction or consumption of platelets. The differences in IPF values between Group 1 and 2 were evaluated using Mann-Whitney U test. A p-value ≤0.05 was considered statistically significant. The receiver operating characteristic (ROC) curve was obtained to determine the cutoff value of IPF for differentiating thrombocytopenia caused by increased peripheral destruction of platelets from thrombocytopenia caused by bone marrow failure/suppression. Cases in both the etiological groups were followed up wherever possible for estimating the platelet recovery by measuring IPF.
The study was performed after due approval from the Human Ethics Committee of the institute “Institutional Human Ethics Committee AIIMS, Bhopal.” All the methods were performed in accordance with the relevant guidelines and regulations.
All the information collected from the cases and controls was kept confidential.
Observations and Results
A total of 61 cases with thrombocytopenia were included in the study. The age of the cases ranged from 7 to 67 years with a median age of 29 years. There were 28 females (46%) and 33 males (54%) in the study group. Along with the cases 101 controls with normal platelet count were also included in the study. The age of the controls ranged from 7 to 65 years with a median age of 27 years. The cases and controls were matched for age and gender. The mean platelet count of cases was 47.3 ± 32.5 × 103/μL and of controls was 254.7 ± 71.1 × 103/μL. The mean IPF of the cases was 8.9 ± 7.6% and of controls was 2.4 ± 1.8% (►Table 1).
| Platelet count (×103/μL) | IPF (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Median | Range | Mean | Standard deviation | Median | Range | |
| Cases | 47.3 | ±32.5 | 48.0 | 3–96 | 8.9 | ±7.6 | 6.6 | 0.1–23.5 |
| Controls | 254.7 | ±71.1 | 252.0 | 147–379 | 2.4 | ±1.8 | 1.8 | 0.7–5.7 |
| p-Value | 0.001 | 0.001 | ||||||
Abbreviation: IPF, immature platelet fraction.
A statistically significant difference was found in the platelet count and IPF of cases and controls with a p-value of 0.001. The normal reference range for IPF was calculated from the findings of 101 controls and found to be ranging from 0.7 to 5.7%. A value of IPF above 5.7% was considered abnormally raised. The IPF was significantly higher in cases with thrombocytopenia than in healthy controls.
Out of 61 cases the final diagnosis was available for 54 cases whereas for seven cases the final diagnosis was not known. The final diagnosis included 13 cases of megaloblastic anemia, 10 cases of dengue, eight cases of hypoproliferative marrow diagnosed with bone marrow biopsy, six cases each of malaria and viral fever, five cases of aplastic anemia, three cases of ITP, two cases of chronic lymphocytic leukemia (CLL), and one case of acute leukemia.
Based on the pathogenesis of thrombocytopenia the 54 cases with final diagnosis were divided into two groups: Group 1 with 29 cases having decreased platelet production due to bone marrow failure/suppression (including megaloblastic anemia, hypoproliferative marrow, aplastic anemia, and acute leukemia) and Group 2 with 25 cases having increased peripheral destruction or consumption of platelets (including dengue, malaria, viral fever, ITP, and CLL). The mean platelet count of Group 1 was 42 ± 35 × 103/μL and that of Group 2 was 56 ± 29 × 103/μL. The mean IPF of the Group 1 was 4.6 ± 4.5% and of Group 2 was 13.4 ± 7.1% (►Table 2). A statistically significant difference was found in the IPF values of Groups 1 and 2 with a p-value of 0.001. The IPF was significantly higher in cases with increased destruction of platelets than in cases with decreased production of platelets.
| Cases | IPF (%) | |||
|---|---|---|---|---|
| Mean | Standard deviation | Median | Range | |
| Group 1 | 4.6 | ±4.5 | 3.7 | 0.1–16.4 |
| Group 2 | 13.4 | ±7.1 | 12.98 | 4.3–23.5 |
| p-Value | 0.001 | |||
Abbreviation: IPF, immature platelet fraction.
The optimal IPF value for discriminating between Groups 1 and 2 was determined using the ROC curve of sensitivity and specificity (►Fig. 1, ►Table 3). An IPF value of 5.95% was optimal cutoff value for distinguishing the two groups of patients with thrombocytopenia with a sensitivity of 88% and specificity of 75.9%.
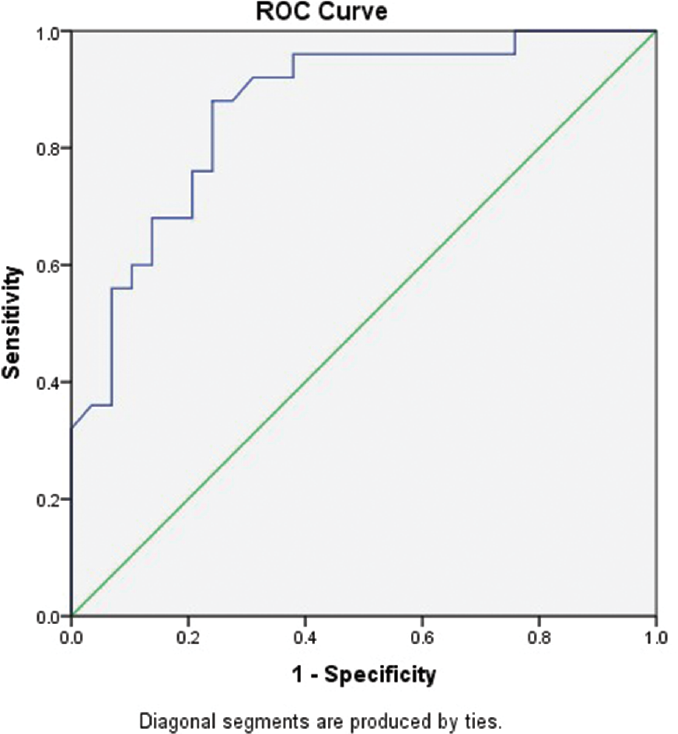
- ROC curve for determining the sensitivity and specificity of IPF to discriminate between Groups 1 and 2. IPF, immature platelet fraction; ROC, receiver operating characteristic.
| Area under curve | Standard error | p-Value | 95% Confidence interval | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| 0.868 | 0.049 | 0.001 | 0.772 | 0.963 |
Abbreviations: IPF, immature platelet fraction; ROC, receiver operating characteristic.
Out of the 61 cases of thrombocytopenia, a 2 to 3 day follow-up was available for only 11 cases and it was observed that the IPF values declined with platelet recovery; however, a statistical analysis was not possible due to small number of cases.
Discussion
IPF reflects the level of thrombopoiesis in thrombocytopenia patients and its measurement is useful in differential diagnosis and analysis of platelet kinetics in thrombocytopenia patients.[8,13-15]
For many years now various studies have shown the advantage of using IPF in differentiating between the underlying pathogenesis of thrombocytopenia and also as an indicator of platelet recovery.[2,4,12]
In the present study an attempt was made to assess and establish the clinical utility of IPF as it is now a part of many upcoming automated hematology analyzers and it provides a rapid, accurate, and valuable information of megakaryocytic activity within minutes.
In the present study the reference range of IPF in healthy controls was estimated to be 0.7 to 5.7% with a mean of 2.4%. The findings of the present study were in concordance with those of the previous studies (►Table 4).[3,4,8,16]
| Reference range | Mean | |
|---|---|---|
| Briggs et al | 1.1–6.7% | 3.4% |
| Dadu et al | 0.7–4.3% | 3.5% |
| Cho et al | 0.4–5.4% | 1.7% |
| Abe et al | 1–10.3% | 3.3% |
| Present study | 0.7–5.7% | 2.4% |
Abbreviation: IPF, immature platelet fraction.
The IPF value was significantly higher in the cases of thrombocytopenia (mean IPF 8.9%) as compared with healthy controls (mean IPF 2.4%) (p-value = 0.001). Briggs et al and Jung et al also reported similar findings in their studies.[2,3] The IPF value was not recordable in three patients with very low platelet counts of < 3 × 103/μL. These patients were later found to be of aplastic anemia and hence they had decreased thrombopoiesis.
In the present study the patients were classified into two groups based on the pathogenesis of thrombocytopenia and it was observed that patients with thrombocytopenia due to increased peripheral destruction of platelets had a more pronounced increase in IPF (13.4%, 4.3–23.5%) as compared with patients with thrombocytopenia due to bone marrow failure/suppression (4.6%, 1–16.4%). This difference in IPF values amongst the two groups was statistically significant (p-value = 0.001). This result indicates that the IPF value is an important parameter for determining the rate of thrombopoiesis and it makes it easier to establish the differential diagnosis of thrombocytopenia at the time of initial diagnosis, hence making the need for bone marrow examination unnecessary. The utilization of IPF in diagnosis of thrombocytopenia helps in discriminating the peripheral destruction of platelets with markedly increased IPF and active thrombopoiesis from hypoplastic bone marrow with decreased thrombopoiesis. These findings were comparable to the ones in studies done by Naz et al, Jung et al, Cho et al, Briggs et al .[1-3,16,17]
The optimal cutoff value of IPF for differentiating patients with increased peripheral destruction of platelets from those with decreased production of platelets was 5.95% with a sensitivity of 88% and specificity of 75.9%, estimated by ROC curve between the two groups of patients. This result may suggest that thrombocytopenic patients with increased IPF of > 5.95 should not be considered as a candidate for bone marrow failure syndrome. Previous studies by Jung et al, Abe et al, and Sakuragi et al reported the cutoff values of 7.7, 6.15, and 7.3%, respectively, which support the findings in the present study.[2,8,13]
In the present study an effort was made to assess the utility of IPF as a predictor of platelet recovery to avoid platelet transfusion and its associated harmful side effects. Out of 61 cases of thrombocytopenia a follow-up was only available for 11 patients and it was observed that the IPF levels correlated with the platelet recovery, however, enough evidence for the same was not available due to smaller number of cases. In previous studies by Dadu et al and Suman et al it has been reported that IPF aids in predicting platelet recovery in patients with dengue fever.[4,12]
To conclude IPF is a rapid, simple, and inexpensive automated parameter to evaluate rate of thrombopoiesis in the bone marrow which is otherwise troublesome and requires a lot of resources and many invasive tests like bone marrow examination. IPF is thus a novel diagnostic method which can be used to differentiate between patients with thrombocytopenia due to different pathogenesis. It can be measured easily along with the routine blood cell parameters and its measurement is useful in initial evaluation of thrombocytopenia patients. Moreover IPF can potentially be useful as a predictor of platelet recovery in thrombocytopenia patients and may prevent unnecessary prophylactic platelet transfusions.
The study was limited by a small sample size and in future more studies with a larger sample size are required for further determining the clinical utility of IPF.
Source(s) of Support
None.
Presentation at a Meeting
None.
Conflict of Interest
None.
References
- Importance of immature platelet fraction as predictor of immune thrombocytopenic purpura. Pak J Med Sci. 2016;32(03):575-579.
- [CrossRef] [Google Scholar]
- Immature platelet fraction: establishment of a reference interval and diagnostic measure for thrombocytopenia. Korean J Lab Med. 2010;30(05):451-459.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of an immature platelet fraction (IPF) in peripheral thrombocytopenia. Br J Haematol. 2004;126(01):93-99.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the immature platelet fraction as an indicator of platelet recovery in dengue patients. Int J Lab Hematol. 2014;36(05):499-504.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus conference on platelet transfusion, Royal College of Physicians of Edinburgh, 27-28 November 1997. Synopsis of background papers. Br J Haematol. 1998;101(04):609-617.
- [CrossRef] [PubMed] [Google Scholar]
- Automated blood cell counts: state of the art. Am J Clin Pathol. 2008;130(01):104-116.
- [CrossRef] [PubMed] [Google Scholar]
- Establishing biological reference intervals for novel platelet parameters (immature platelet fraction, high immature platelet fraction, platelet distribution width, platelet large cell ratio, platelet-X, plateletcrit, and platelet distribution width) and their correlations among each other. Indian J Pathol Microbiol. 2014;57(02):231-235.
- [CrossRef] [PubMed] [Google Scholar]
- et al A simple technique to determine thrombopoiesis level using immature platelet fraction (IPF) Thromb Res. 2006;118(04):463-469.
- [CrossRef] [PubMed] [Google Scholar]
- et al Flow cytometric analysis of reticulated platelets: evidence for a large proportion of non-specific labelling of dense granules by fluorescent dyes. Br J Haematol. 1998;100(02):351-357.
- [CrossRef] [PubMed] [Google Scholar]
- et al Correlation between immature platelet fraction and reticulated platelets. Usefulness in the etiology diagnosis of thrombocytopenia. Eur J Haematol. 2010;85(02):158-163.
- [CrossRef] [PubMed] [Google Scholar]
- Time course of immature platelet count and its relation to thrombocytopenia and mortality in patients with sepsis. PLoS ONE. 2018;13(01):e0192064.
- [CrossRef] [PubMed] [Google Scholar]
- The use of immature platelet fraction to predict time to platelet recovery in patients with dengue infection. Paediatr Int Child Health. 2020;40(02):124-128. DOI: 10.1080/20469047.2019.1697574
- [CrossRef] [PubMed] [Google Scholar]
- et al Immature platelet fraction (IPF) as a predictive value for thrombopoietic recovery after allogeneic stem cell transplantation. Int J Hematol. 2018;107(03):320-326.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the immature platelet fraction in the diagnosis and prognosis of childhood immune thrombocytopenia. Platelets. 2015;26(07):645-650.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of the immature platelet fraction in pediatric immune thrombocytopenia: differentiating from bone marrow failure and predicting bleeding risk. Pediatr Blood Cancer. 2018;65(02):e26812.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical usefulness of the simple technique to diagnose thrombocytopenia using immature platelet fraction. Korean J Lab Med. 2007;27(01):1-6.
- [CrossRef] [PubMed] [Google Scholar]
- et al Immature platelet fraction: a useful marker for identifying the cause of thrombocytopenia and predicting platelet recovery. Medicine (Baltimore). 2020;99(07):e19096.
- [CrossRef] [PubMed] [Google Scholar]





