Translate this page into:
Immunohistochemistry-based comparative study in detection of Hirschsprung's disease in infants in a Tertiary Care Center
Address for correspondence: Dr. Chhanda Das, 31 Eastern Park, Santoshpur, Kolkata - 700 075, West Bengal, India. E-mail: chhhdas@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
BACKGROUND:
Hirschsprung's disease (HD) is the major cause of pediatric intestinal obstruction with a complex pattern of inheritance. The absence of ganglion cells along with an analysis of hypertrophy and hyperplasia of nerves in the nerve plexus of submucosa and muscularis mucosae is regarded as a potential hallmark for its diagnosis.
AIMS AND OBJECTIVES:
This study was undertaken to ascertain the (1) clinical profile, (2) mode of presentation, and (3) to compare the role of calretinin immunostaining with acetylcholinesterase in the diagnosis of HD.
MATERIALS AND METHODS:
This prospective and observational study was conducted in the Department of Pathology, IPGME & R from June 2014 to May 2015. One hundred and four patients clinically and radiologically diagnosed with HD underwent surgery were included in the study. The data of every patient including age, sex, and presenting symptoms were recorded. Eventually, histopathological, calretinin, and acetylcholinesterase immunohistochemical examination were done.
RESULTS:
Total numbers of cases studied were 104, which aged between 0 days and 365 days. Male preponderance (76.92%) was noted. The overall sensitivity, specificity, positive, and negative predictive value of acetylcholinesterase were 100%, 86.44%, 84.91%, and 100%, respectively. The concordance of detection of ganglion cells and nerve fibers, and thereby diagnosis of Hirschsprung's and non-HD using calretinin and the gold standard was statistically in strong agreement (κ = 0.749, 95% confidence interval: 0.635–0.863).
CONCLUSIONS:
Calretinin stands out as the single and indispensable tool that differentiates HD from other mimickers.
Keywords
Acetylcholinesterase
calretinin
Hirschsprung's disease
infants
Introduction
Hirschsprung's disease (HD), a genetic disorder with a complex pattern of inheritance, is the major cause of pediatric intestinal obstruction.[1] Failure of migration of ganglion cells from neural crest leads to the development of aganglionic segments which varies from ultra-short to total intestinal involvement.[23] According to a recent survey, the incidence of HD is 1 in 5343 live births with male preponderance.[4]
Qualitative and morphological evaluation of ganglion cells along with an analysis of hypertrophy and hyperplasia of nerves in the nerve plexus of submucosa and muscularis propria are regarded as a potential hallmark for diagnosis of HD.[56] Reliable diagnosis in hematoxylin and eosin (H and E) staining depends on observer's ability to analyze several serial sections.[7] To maximize the diagnostic accuracy, especially in ultra-short segment HD, additional laboratory methods were introduced including enzyme histochemistry and immunohistochemistry (IHC) using neuronal markers. Aganglionic segments reveal increased enzyme histochemical acetylcholinesterase reaction in parasympathetic nerve fibers of lamina propria.[8910] However, an alternative neuronal marker is essential to reduce false negative cases.[1112] Qualitative evaluation of ganglion cells by calretinin IHC yielded no misdiagnosis or major discrepancies between observers.
Calretinin, a calcium-binding protein, plays an important role in the diagnosis of the aganglionic segment in HD. Calretinin expression is observed in ganglionic cells within the submucosal and myenteric plexus of normal bowel whereas aganglionic regions of HD lack calretinin expression.[13] In our study, we attempt to find the role of this novel method in the diagnosis of HD and compare the same with the acetylcholinesterase immunohistochemical technique considering H and E as the gold standard.
Materials and Methods
After obtaining Ethical Committee approval, this prospective and descriptive study was conducted in the Department of Pathology in collaboration with Department of Pediatric Surgery. Two pathologists conducted the histopathological and immunohistochemical interpretation blindly without knowing the clinical diagnosis. The final histological diagnosis was then compared with the immunohistochemical findings.
This study was undertaken from June 2014 to May 2015 taking 104 infants (age range: 0–365 days) with clinically and radiologically suspected HD. Radiological diagnosis was made after evaluation of the following parameters: (1) Transitional zone, (2) rectosigmoid index, (3) irregular contraction, (4) cobblestone appearance, (5) filling defect, and (6) lack of meconium defecation during the first 48 h. Anorectal manometry was also done in radiologically unequivocal cases. Patients with inadequate clinical information were not included in this study. After taking valid consent from the parents, a detailed history was taken, and clinical examination along with any presence of malformation was noted preoperatively.
The biopsy specimens were sent for histopathological examination. Two hundred and fifteen blocks (188 from biopsies and 27 from total colectomy specimens) were sectioned and stained with H and E stain. Representative sections from spastic segment, transitional zone, and ganglionic region were embedded. One distal colon full thickness sample and one proximal colon full thickness sample with microscopic evidence of ganglion cells were taken as positive controls. One slide of a previously diagnosed case of HD was taken as negative control.
All slides were examined under the light microscope by two observers. Positive interpretation (non-Hirschsprung's disease [NHD]) was based on the identification of at least one ganglion cell in representative sections. The absence of ganglion cells with or without hypertrophic nerve fiber in submucosa and muscularis propria is considered as HD.
Unbinding and comparing with immunohistochemical findings
One hundred and five blocks were selected for immunohistochemical staining. Blocks without representative tissue or containing only superficial mucosa were eliminated. Exactly 3 μm sections were taken on poly-l-lysine-coated slides. Ready-to-use Calretinin from cell Dako (FLEX Monoclonal Mouse Anti-Human Calretinin Clone DAK-Calret 1) was used for immunostaining after deparaffinization, clearing, and rehydration. The antibody used predominantly stained cytoplasm and nuclei of ganglion cells. Calretinin was considered as positive if any specific staining was present either within the submucosal or the myenetric plexus. Mast cell staining was considered as internal positive control.
Acetylcholinesterase (AChE) is a marker of cholinergic nerves. For AChE IHC ready to use (BIORBYT: Protein & Antibodies Manufacturer &Supp, Headquarters Cambridge) AChE antibody (orb38623) was used on conventional formalin-fixed, paraffin-embedded tissue. It is a rabbit polyclonal anti-human immunoglobulin G. Detection of immunoreactive nerve bundles in the submucosa and muscularis externa along with aganglionosis is a strong indicator of HD [Figure 1]. Thickened nerves are detected by Labomed Pixel Pro™ Software using ruler and diameter more than 40 µm is considered as thickened nerve.
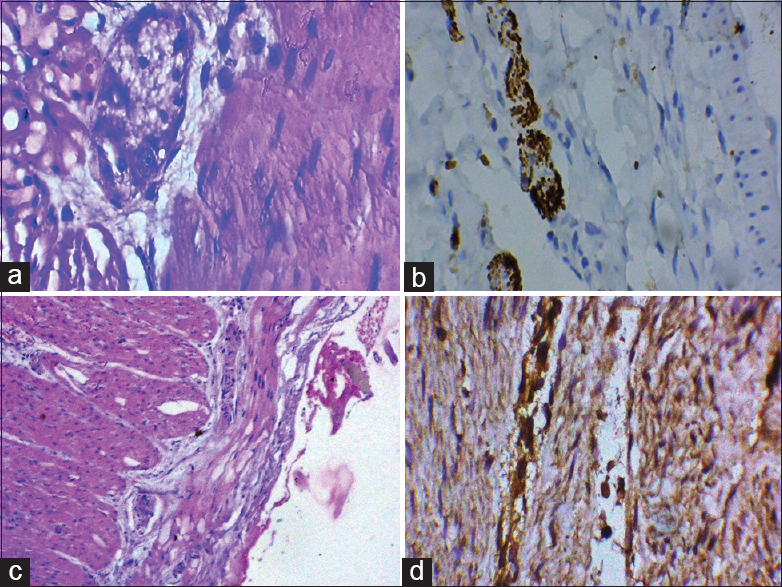
- (a) Mature ganglion cells in myenteric plexus (×400); (b) calretinin positive immunohistochemistry; (c) hypertrophic nerve fibers; (d) acetylcholine positive nerve fiber
Statistical analysis
Histopathological diagnosis was taken as the “gold standard” and the other procedures were statistically analyzed using Chi-square test, sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy, etc. Software used in statistical analysis of our study was MedCalc version 11.6 (MedCalc Software 2011, Mariakerke, Belgium). Agreements between two observers were analyzed.
Results
A total of 104 patients considered for the specified period that satisfied the inclusion and exclusion criteria were enrolled in our study.
Clinical profile
A total no of 104 cases were included in this study of which 80 patients were male (76.92%) and the rest 24 patients (23.08%) were female [Figure 2]. The age ranged was from 0 days to 1 year. Sixty-two (59.62%) of 104 cases were neonates. Five (4.8%) were from 0 days to 2 days, 57 (54.80%) were from 3 days to 28 days and remaining 42 (40.4%) presented 29 days–1 year. Delayed passage of meconium (37.5%) was the main diagnostic symptom in most cases, followed by abdominal distension (32.7%). Other presenting symptoms were gastrointestinal perforation, bilious vomiting, and meconium ileus [Table 1].
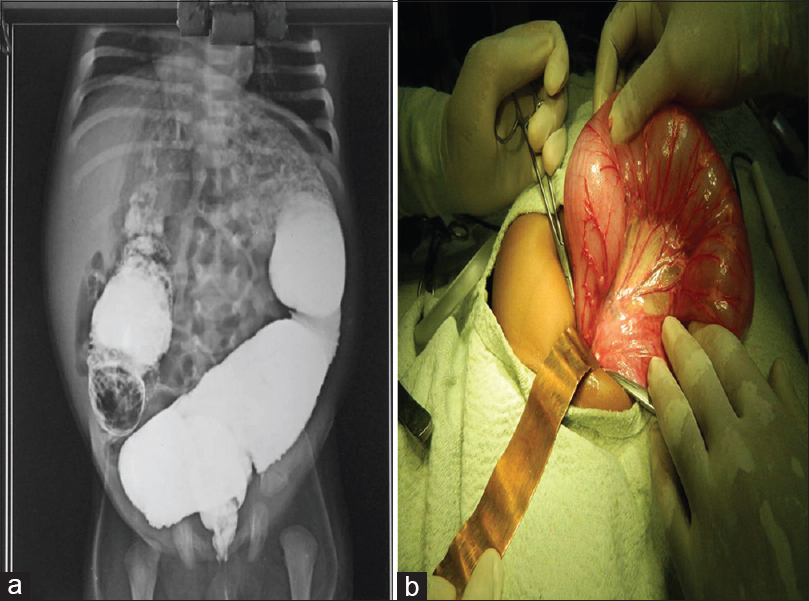
- (a) Transitional zone in barium enema; (b) intraoperative dilated colon

Histopathological examination
To define the site of involvement in Hirschsprung's disease, we subdivided as follows: (1) Rectosigmoid (short segment), (2) long segment, (3) ultra-short segment, (4) total colonic, and (5) total intestinal.
Table 2 shows that the most common site of involvement was a rectosigmoid region (79.8%).

Two hundred and fifteen histopathological slides were examined by two pathologists blindly. A discrepancy was noted in only one case. High consensus was found between two observers (P < 0.001).
Acetylcholinesterase immunohistochemistry
Regarding AChE IHC examination, 105 slides from 104 patients were examined. Satisfactory concordance was obtained between H and E reports and AChE study. Overall sensitivity, specificity, positive, and negative predictive value were 100%, 86.44%, 84.91%, and 100%, respectively [Table 3].

Immunohistochemistry profiling of calretinin
One hundred and five blocks were selected for calretinin IHC. All representative areas including lamina propria, submucosa, and muscularis mucosae were examined for detection of calretinin positivity by two pathologists blindly. Satisfactory concordance was obtained between H and E reports and calretinin study. Overall sensitivity, specificity, positive, and negative predictive value were 100%, 92.73%, 92.45%, and 100%, respectively [Table 4].

Cross tabulation of findings obtained by the two observers of both immunohistochemical staining on 105 slides from 104 patients with suspected Hirschsprung's disease
The calretinin results were compared with the gold standard. The concordance of detection of ganglion cells and nerve fibers, and thereby diagnosis of Hirschsprung's and NHD using calretinin and the gold standard was statistically in strong agreement (κ = 0.749, 95% confidence interval 0.635–0.863) [Table 5, correation of both IHC].

Discussion
Being the major cause of pediatric intestinal dysmotility, priority is given to the early diagnosis of HD which is characterized by aganglionosis and cholinergic hyperactivity.[14] Diagnostic modalities are broadly divided into (1) clinical examination, (2) radiological examination, and (3) histopathological examination of the biopsy specimens. De Lorijn et al. stated the highest sensitivity (93%) and specificity (100%) of rectal suction biopsies among all diagnostic methods.[15] Biopsy specimens can be subdivided into the following categories depending on depth of the biopsies - biopsy specimens containing mucosa and muscularis mucosae; mucosa and superficial muscularis mucosae; mucosa, muscularis mucosae, submucosa, and muscularis propria; muscularis propria only; mucosa, muscularis mucosae, and submucosa; and mucosa, muscularis mucosae, and superficial submucosa.[16] In conventional H and E stain, NHD and HD are categorized depending on the presence and absence of ganglion cells in one or more tissue sections.[17] False positive interpretation of HD is usually contributed by the following factors (1) minimally appreciable submucosa depending on the biopsy obtained, (2) inappreciable and infrequent ganglion cells in submucosa, (3) difficulty in identification of immature ganglion cells specially in neonates, and (4) observers’ error due to lack of experience.
To minimize false positive cases of HD, especially in case of hypoganglionosis, other ancillary investigations such as enzyme histochemistry and IHC were introduced. The principle of histochemistry is based on detection of enzyme AChE secreted by extrinsic preganglionic parasympathetic nerves that accumulate in excess in muscularis mucosae and circular muscle.[18] In spite of several advantages of AChE histochemistry, technical difficulties and pitfalls were reported by several authors. The requirement of fresh frozen tissue is considered as one of main obstacles in routine laboratory method followed by certain diagnostic pitfalls like an age-pattern relationship (Pattern I).[19]
In our study, we selected AChE IHC to overcome the disadvantages of histochemistry. We also compare the utilization of AChE IHC along with calretinin which is a general neuronal marker of both mature and immature neurons. Calretinin IHC was found superior to AChE IHC in our study considering the following aspects:
-
Being a calcium binding protein, calretinin express in submucosal and myenteric plexus of the ganglionic bowel. Qualitative evaluation of calretinin is simple and specific. However, interpretation in case of AChE IHC depends on the level of parasympathetic hypersecretion
-
Monoclonal mouse anti-human calretinin antibody stained cytoplasm and nuclei of ganglion cells. Although AChE is considered as usual marker of cholinergic nerves, many studies revealed that AChE-stained noncholinergic peripheral neurons also[20]
-
Calretinin expression can be assessed in biopsy containing mucosa and muscularis mucosae only, but submucosal tissue is essential in evaluation of AChE IHC[21]
-
In our study, we came across certain difficulties in AChE IHC interpretation, especially in neonates. However, calretinin expression is uniform in all infants
-
Calretinin IHC kits stored at 4°C but −20°C temperature is required in case of AChE antibody storage
-
In our laboratory, the use of AChE antibody is restricted to the diagnosis of HD whereas calretinin IHC kit is kept as a regular stain.
We found calretinin negative staining in one case of ultra-short segment HD in our study. However, AChE IHC plays a complementary role in this case revealing hypertrophic nerve fibers.
Conclusion
In spite of various immunohistochemical markers such as cholinergic marker, neuropeptides, synaptic markers, and supporting cell markers, calretinin stands out as the single and indispensable tool that differentiates HD from other mimickers such as pseudo-Hirschsprung's disease, megacystis-microcolon hypoperistalsis syndrome, and visceral neuropathies and myopathies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Enteric nervous system: Development and developmental disturbances – Part 2. Pediatr Dev Pathol. 2002;5:329-49.
- [Google Scholar]
- Enteric nervous system: Development and developmental disturbances – Part 1. Pediatr Dev Pathol. 2002;5:224-47.
- [Google Scholar]
- Hirschsprung's disease in Japan: Analysis of 3852 patients based on a nationwide survey in 30 years. J Pediatr Surg. 2005;40:197-201.
- [Google Scholar]
- Biopsy diagnosis of Hirschsprung's disease and related disorders. Curr Top Pathol. 1990;81:257-75.
- [Google Scholar]
- A modified technique for the diagnosis of Hirschsprung disease from rectal biopsies. Natl Med J India. 2003;16:245-8.
- [Google Scholar]
- Controversies concerning diagnostic guidelines for anomalies of the enteric nervous system: A report from the fourth International Symposium on Hirschsprung's disease and related neurocristopathies. J Pediatr Surg. 2005;40:1527-31.
- [Google Scholar]
- Problems and advantages of acetylcholinesterase histochemistry of rectal suction biopsies in the diagnosis of Hirschsprung's disease. J Pediatr Surg. 1990;25:520-6.
- [Google Scholar]
- The loss of calretinin expression indicates aganglionosis in Hirschsprung's disease. J Clin Pathol. 2004;57:712-6.
- [Google Scholar]
- Histological diagnosis and differential diagnosis. In: Holschneider AM, Puri P, eds. Hirschsprung's Disease and Allied Disorders. Amsterdam: Harwood; 2000. p. :252-65.
- [Google Scholar]
- Diagnosis of Hirschsprung's disease: A prospective, comparative accuracy study of common tests. J Pediatr. 2005;146:787-92.
- [Google Scholar]
- The identification of ganglion cells in Hirschsprung disease by the immunohistochemical detection of ret oncoprotein. Am J Clin Pathol. 2006;126:49-54.
- [Google Scholar]
- Role of syanptophysin in leveling circumferential full thickness (doughnut) bowel. Indian J Pathol Microbiol. 2012;55:s10.
- [Google Scholar]
- Selective demonstration of mural nerves in ganglionic and aganglionic colon by immunohistochemistry for glucose transporter-1: Prominent extrinsic nerve pattern staining in Hirschsprung disease. Arch Pathol Lab Med. 2000;124:1314-9.
- [Google Scholar]
- New classification of histochemical staining patterns of acetylcholinesterase activity in rectal suction biopsy in Hirschsprung's disease. J Med Assoc Thai. 2000;83:1196-201.
- [Google Scholar]
- Identification of cholinergic neurons in enteric nervous system by antibodies against choline acetyltransferase. Am J Physiol. 1993;265(5 Pt 1):G1005-9.
- [Google Scholar]
- Calretinin immunohistochemistry versus improvised rapid acetylcholinesterase histochemistry in the evaluation of colorectal biopsies for Hirschsprung disease. Indian J Pathol Microbiol. 2014;57:369-75.
- [Google Scholar]





