Translate this page into:
Klebsiella Pneumoniae in Septicemic Neonates with Special Reference to Extended Spectrum β-lactamase, AmpC, Metallo β-lactamase Production and Multiple Drug Resistance in Tertiary Care Hospital
Address for correspondence: Ms. Shivali V Gajul, E-mail: shivali_gajul@yahoo.co.in
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
β-lactamases viz., extended spectrum β-lactamase (ESBL), AmpC, and metallo β-lactamase (MBL) production in Klebsiella pneumoniae has led to a serious concern about septicemic neonates in Neonatal Intensive Care Units due to high resistance against commonly used antimicrobials.
Purpose:
To study the prevalence of ESBL, AmpC, and MBL production in K. pneumoniae isolates in neonatal septicemia, to check antimicrobial susceptibility to various drugs including tigecycline; and to assess burden of multiple drug resistance (MDR).
Materials and Methods:
Total 24 clinical isolates of K. pneumoniae isolated from 318 blood samples of suspected cases of neonatal septicemia were studied. Isolates were screened for ESBL, AmpC, and MBL production by Clinical and Laboratory Standards Institute (CLSI) disk method, AmpC cefoxitin screen, and imipenem, meropenem, ceftazidime disk screen respectively; and confirmation was done by CLSI phenotypic disk confirmatory test, AmpC sterile disk method, and imipenem ethylenediamine tetracetic acid double disk synergy test respectively. Antimicrobial susceptibility was determined by Kirby–Bauer's disk diffusion method. Efficacy of tigecycline was evaluated using United States Food and Drug Administration guidelines.
Results:
Of the 24 K. pneumoniae isolates, co-production of AmpC + MBL was found in more number of isolates (67%) (P < 0.0001) compared to single enzyme production (ESBL and MBL 8% both, AmpC 12.5%). Rate of resistance for penicillins and cephalosporins was highest. Susceptibility was more for imipenem, co-trimoxazole, and meropenem. Nonsusceptibility to tigecycline was low (21%). A total of 23 (96%) isolates were MDR.
Conclusions:
Routine detection of ESBL, AmpC, and MBL is required in laboratories. Carbapenems should be kept as a last resort drugs. Trend of tigecycline susceptibility has been noted in the study. Continued monitoring of susceptibility pattern is necessary to detect true burden of resistance for proper management.
Keywords
AmpC
extended spectrum β-lactamase
Klebsiella pneumoniae
metallo β-lactamase
neonatal septicemia
INTRODUCTION
Neonatal deaths account for over a one-third of the global burden of child mortality. Sepsis is the significant cause of morbidity and mortality in neonates. Prematurity, low birth weight and prolonged hospitalization are the predisposing factors for neonatal sepsis. Inadequate space, shortage of staff, high occupation rates, widespread use of antimicrobial agents and increased susceptibility of the population are responsible for early colonization and subsequent infection by virulent strains resulting in high morbidity and mortality.[1] Approximately one million deaths a year occurring in the neonatal period (0–28 days) are caused by infection, accounting for over 25% of global neonatal deaths and 99% of these deaths occur in developing countries.[2] The World Health Organization estimates that one million deaths per year are due to neonatal sepsis and that 42% of these deaths occur in the 1st week of life.[3]
Most common bacterial organisms responsible for neonatal septicemias in developed countries include coagulase-negative Staphylococcus and group B Streptococcus while in developing countries like Pakistan, India, Nigeria, Bangladesh, are Escherichia coli, Klebsiella, Enterobacter etc.[4] According to the data of National Neonatal Perinatal Database 2005, 40% of neonatal deaths were ascribed to sepsis in India.[5] Klebsiella pneumoniae was the most frequently isolated pathogen (32.5% and 30.1%) among both intramural and extramural admissions.[6]
In developing countries, multiple drug resistant (MDR) organisms causing neonatal sepsis are increasing, and K. pneumoniae is often reported in this context. K. pneumoniae is resistant to numerous antimicrobial agents by different mechanisms, including expression of extended spectrum β-lactamases (ESBLs), AmpC β-lactamases, 16S rRNA methylases, aminoglycoside modifying enzymes and carbapenemases.[7] Emergence of ESBLs is a vital factor in the treatment of infections associated with sepsis. ESBL producing isolates show resistance to β-lactam antibiotics, including third-generation cephalosporins, in addition; they often exhibit resistance to other classes of drugs such as aminoglycosides, co-trimoxazole, tetracycline and fluoroquinolones. Thus, they pose an intimidating challenge with limited therapeutic options, particularly in resource-challenged countries.[5]
AmpC β-lactamases are cephalosporinases that are poorly inhibited by clavulanic acid and can be differentiated from ESBLs by their ability to hydrolyze cephamycins. These days, AmpC is also being found in K. pneumoniae isolates. The increased prevalence of both ESBLs and AmpC in bacterial isolates creates prerequisite for laboratory testing methods that can accurately detect the presence of these enzymes.[8]
Recently, metallo β-lactamases (MBLs) have emerged as one of the most feared resistance mechanisms due to their ability to hydrolyze virtually all β-lactam agents including carbapenems. Its spread on highly mobile gene elements in nosocomial pathogens limits the therapeutic options.[9] It has thus become essential to be alert about the trend in susceptibility patterns of organisms to save the therapies.
To combat the escalating rate of drug resistance, newer drugs like tigecycline are being discovered. Tigecycline, an expanded broad-spectrum antibiotic; is a semi-synthetic glycylcycline derived from minocycline and has activity against Gram-negative pathogens that are refractory as a result of both efflux and ribosomal protection mechanisms. In addition, organisms that are resistant to other antimicrobial classes do not exhibit cross-resistance to tigecycline, affirming its potential therapeutic use for ESBL and carbapenemase producing Enterobacteriaceae infections.[10] Due to the promising microbiological, pharmacodynamic and pharmacokinetic profile against significant pathogens, its role is being evaluated for empirical therapy in seriously ill-patients.
The present study was conducted (1) To know the incidence of ESBL, AmpC, and MBL production among clinical isolates of K. pneumoniae in neonatal sepsis, (2) To identify the susceptibility pattern to the number of antimicrobials including carbapenems and especially tigecycline, (3) To assess the burden of MDR. To the best of our knowledge, this is the first comprehensive study of three different β-lactamases producing K. pneumoniae implicated in neonatal sepsis in a developing country setting, with special reference to MDR and tigecycline susceptibility.
MATERIALS AND METHODS
The present study was carried out from January 2010 to February 2012 in Microbiology Department of BLDEU's Shri B. M. Patil Medical College, Hospital and Research Center, Bijapur. A total of 318 blood samples from cases of neonatal septicemia sent for blood culture were included in the study. Briefly, 1–2 ml of blood was collected for culture into 10 ml of soybean casein digest broth. Cultures were processed in BacT/ALERT 3D system (bioMérieux, Marcy l’Etoile, France). These broths were incubated in system's incubator at 37°C under aerobic conditions for 7 days and observed for the growth of organisms. Any sign of growth was followed by sub-culture on MacConkey's agar and blood agar plates (HiMedia Laboratories, Mumbai) and identified using standard microbiological techniques.
Detection of extended spectrum β-lactamase
The screening for ESBL production was done as per Clinical, and Laboratory Standards Institute (CLSI) recommended method.[11] Isolate showing inhibition zone of ≤17 mm for cefpodoxime (10 μg), ≤22 mm for ceftazidime (30 μg), ≤27 mm for aztreonam (30 μg), ≤27 mm for cefotaxime (30 μg), ≤25 mm for ceftriaxone (30 μg) was taken for ESBL confirmation. The confirmation was done by CLSI phenotypic disk confirmatory test using disks of ceftazidime (30 μg) and ceftazidime-clavulanic acid (30 μg/10 μg). Both the disks were placed at least 20 mm apart, centre to centre on Mueller-Hinton agar plate and incubated over night at 37°C. A zone difference of ≥5 mm around ceftazidime and ceftazidime-clavulanic acid was taken as ESBL positive. K. pneumoniae ATCC 700603 and E. coli ATCC 25922 were used as positive and negative control respectively.[11]
Detection of AmpC β-lactamase
All the isolates were screened for AmpC β-lactamase by Kirby–Bauer's disk diffusion method using cefoxitin (30 μg) disk. An isolate demonstrating reduced susceptibility (<18 mm zone of inhibition) to cefoxitin was taken as screen positive. Screen positive isolates were further confirmed by AmpC sterile disk test. E. coli ATCC 25922 was used as control.[12]
Detection of metallo β-lactamase
Organisms showing resistance to any of the three antimicrobials viz., imipenem (10 μg), meropenem (10 μg), and ceftazidime (30 μg) was considered as a screen test positive. Screen positive isolates were confirmed by imipenem ethylenediamine tetracetic acid double disk synergy test Pseudomonas aeruginosa ATCC 27853 was used as control.[13]
Antimicrobial susceptibility testing
Antibiotic susceptibility testing was done by Kirby–Bauer's disk diffusion method as per CLSI guidelines.[11] Antimicrobial disks used in the study were purchased from HiMedia Laboratories, Mumbai. Efficacy of tigecycline was evaluated by using United States Food and Drug Administration (US FDA) guidelines since, interpretive criteria for this drug was not proposed by CLSI (susceptible ≥19 mm, nonsusceptible ≤14 mm).[14] Quality control was achieved by using standard strain of E. coli ATCC 25922.
RESULTS
During the study period, a total of 318 neonates were suspected to have sepsis. Of these, 114 (36%) cases were culture positive. Among these culture positives, 24 (21%) isolates were identified as K. pneumoniae. Screening for ESBL gave 23 isolates positive, for AmpC it was 20 isolates and for MBL, imipenem gave 2, meropenem gave 14 and ceftazidime gave 23 isolates positive.
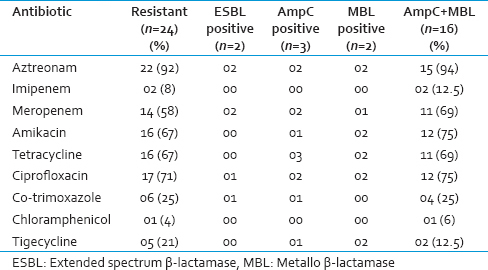
Results of confirmatory tests of three β-lactamases are given in Table 1. Among 24 isolates, all except one isolate produced either type of β-lactamase. AmpC production was more (12.5%) compared to ESBL and MBL production (8% both), and the occurrence of co-production of AmpC + MBL was found in 16 isolates (67%).

Results of antimicrobial susceptibility to cephalosporins and other antimicrobials are given in Tables 2 and 3. All the isolates except one, that is, total 23 (96%) isolates were found to be multiple drug resistant, as they were resistant to more than two drug classes as per CDC guidelines.[15]

DISCUSSION
Septicemia is the major problem in the neonatal population around the world. Timely detection and initiation of proper antimicrobial therapy can be helpful in saving the life. In our study, the incidence of neonatal sepsis confirmed by blood culture was 36% (114/318) and we could obtain 21% (n = 24) isolates of K. pneumoniae. There has been a wide variation in the growth positivity and the prevalence of K. pneumoniae isolates. Culture positivity from 37% to 53% and the rate of prevalence of K. pneumoniae from 26% to 48% has been reported by various authors.[91617]
As the report of blood culture and antibiotic susceptibility are available after 72 h or more, any delay in the initiation of correct empirical therapy or improper choice of antimicrobials cannot be justified. A common practice is to use ampicillin and an aminoglycoside or a third-generation cephalosporin in neonatal sepsis. Widespread use of third-generation cephalosporins and aztreonam is believed to be the major cause of the mutations in TEM and SHV enzymes that has led to the emergence of the ESBLs.[8] ESBL screen test gave 23 (96%) isolates positive. Study done by Modi et al., reported 89% positive in ESBL screening.[8] ESBL production was confirmed in only 2 isolates [Table 1] that is, its prevalence in Neonatal Intensive Care Unit was 8.3%, which was remarkably less compared to other studies (31–47.8%).[5917]
There has been a paucity of data about the prevalence of AmpC producing strains in India, and very little information is available in neonatal group. In the present study 20 (83%) isolates were AmpC screen positive. Out of these, only 3 (12.5%) isolates were positive by confirmatory test [Table 1]. The rate of AmpC production was more compared to other reports from India suggesting broader spectrum of resistance in our isolates.[1816]
The production of MBLs in strains largely limits therapeutic options. In screening of MBL, we found 2 (8.3%) isolates resistant to imipenem, 14 (58%) isolates resistant to meropenem, and 23 (96%) isolates resistant to ceftazidime. Confirmatory test gave positive results for only 2 (8.3%) isolates [Table 1]. In a study carried out by Vinod Kumar et al., 20% resistance to imipenem and 17% rate of MBL production was reported.[9] Similarly, Kamble et al., reported 20% MBL production.[17]
In our study, co-production of two β-lactamases in single isolates was observed. This was an alarming finding with very large number, that is, 16 isolates (67%) producing both AmpC + MBL [Table 1]. There was a significant difference between β-lactamase productions with a maximum number of isolates co-producing two enzymes (χ2 = 32.191, P < 0.0001). One prominent finding was that, among these 16 isolates; 13 isolates were sensitive to imipenem, 4 isolates were sensitive to meropenem, and 2 isolates were intermediate sensitive to meropenem. These results indicate that if susceptibility reports are being referred for treatment without laboratory testing of β-lactamases, it can result in serious therapeutic failures. Isolates producing more than one β-lactamase have been reported in few studies, however rarely any study had documented multiple productions to a larger extent in the neonatal population. Study done by Singh et al., reported co-production of ESBL + AmpC 14.8%, MBL + AmpC 18.5%, and ESBL + MBL 11.1% in neonatal sepsis.[16]
In most centers β-lactamase production is not routinely tested which ultimately results in the dissemination of β-lactamase producing strains in hospitals, and it remains undetected for longer periods. Inappropriate usage of antimicrobials leads to escalating percentage of β-lactamase production. Therefore, preventive antibiotics should be used as little as possible and specific therapeutic antibiotics should be used for short period as suggested by Singh et al.,[16] In conditions, wherein the use of antibiotics is necessary, rotation of antibiotic regimens is suggested.
Antimicrobial susceptibility profile of total 24 isolates and selectively of the isolates producing three β-lactamases and AmpC + MBL showed a high degree of resistance to antimicrobials. The resistance for penicillins, aztreonam, and cephalosporins were highest (92–100%) [Table 2]. In neonatal sepsis, the third-generation cephalosporins have been used extensively as a first-line antibiotic; as a result of which they are rendered uselessly. Our isolates showed least resistance for imipenem, co-trimoxazole, followed by meropenem (8–12.5%, 25% and 58–69% respectively) [Table 3]. The rate of resistance to the various drugs was in co-ordinance with other studies.[581617] Amikacin and ciprofloxacin are good alternatives, and they will also provide some economic relief to the poor patients.
In our study, the lesser number of ESBL positivity may be due to high-level expression of AmpC or MBL, which can mask the phenotype of ESBL. This masking effect can be detected by methods using boronic acid, however it has been stated that confirmation of ESBL production by clavulanic acid inhibition can be difficult not only because the activity of the β-lactamase varies with different substrates, but also because organisms may contain additional resistance mechanisms that can mask the presence of ESBL activity. These could include AmpC-type enzymes, porin changes, and TEM and SHV β-lactamases that are no longer inhibited by clavulanic acid due to mutations in the coding sequences.[18]
Though molecular techniques are the gold standard, it is difficult for most of the diagnostic laboratories to do on a routine basis. On the other hand, although phenotypic methods give little imprecise results, they are widely being used because of their simplicity and cost-effectiveness. ESBL detection by only CLSI method and lack of molecular data is the limitation of the study.
In the current scenario of burden of resistance, development of tigecycline drug is a significant advancement. In the total 24 study isolates, nonsusceptibility to tigecycline was low (5 isolates, 21%) [Table 3]. Two ESBL producing isolates were susceptible (0% resistance) to tigecycline whereas, one AmpC producing, two MBL producing, and two AmpC + MBL co-producing isolates were nonsusceptible (33%, 100%, 12.5% resistance respectively). This study showed good activity of tigecycline against K. pneumoniae producing ESBL, AmpC, as well as both AmpC + MBL co-producing isolates. It lost its activity against MBL producing isolates, although the numbers of MBL producers were less to arrive at the definite conclusion. The clinical efficacy of tigecycline in neonatal sepsis has not yet been established. In vitro evaluation of its efficacy in ESBL and MBL producing isolates in neonatal septicemia have been reported by Roy et al., in two different studies.[719]
Tigecycline has not been approved by the FDA for the treatment of bloodstream infections.[1020] It has unusual pharmacokinetic properties, resulting in low blood concentrations but somewhat higher tissue levels.[21] Hence, its extensive tissue distribution allows for eradication of the source of secondary bacteremia that is likely to result in the clinical resolution.[20] However, more clinical experience with this drug is required to understand its role in the treatment of infections caused by carbapenemase producing K. pneumoniae[10] and clinicians should be more cautious when implementing tigecycline for the treatment of suspected or proven bacteremia, and its use should be guided preferably by the minimum inhibitory concentrations (MIC).[20] Hence the MIC study has been advocated unanimously for evaluating its efficacy. However, some studies have underlined the convenient and cost-effective disk diffusion method and proved its efficacy over the laborious MIC methods.[102223]
Studies regarding the comparison of MIC and disk diffusion methods for tigecycline done by various authors have reported good correlation (92–100%).[102223] Moreover, Kumar et al., has stated that, the microbiology laboratories might use the relatively easier method of disc diffusion, when compared to the comparatively tedious method of MIC determination.[10] Similar to our study, use of disk diffusion method for tigecycline susceptibility has been reported by Filgona et al.[24]
In the present era, the emergence of MDR organisms and their spread in the community is of great concern. Infections by MDR organisms lead to prolonged hospitalization, increased mortality, morbidity and cost of treatment.[14] As per the definition of CDC guidelines formulated for “the management of MDR organisms in healthcare settings,” isolates that were resistant to two or more of the most commonly used antimicrobial classes for the treatment were placed in MDR category.[15] Isolates exhibiting co-resistance to at least any two of the following drugs were considered as MDR and these drugs were: Third generation cephalosporin (cefixime/ceftriaxone/ceftazidime), an aminoglycoside (amikacin), a fluoroquinolone (ciprofloxacin), and a folate pathway inhibitor (co-trimoxazole). In our study, all isolates except one, that is, total 23 (96%) isolates were found to be MDR. Various authors have reported high percentage of MDR in their study.[158] Our findings are in concordance with them.
CONCLUSIONS
In our study, a high prevalence of AmpC + MBL co-producing isolates and MDR isolates shows that there is a grave trouble in the treatment of infections caused by MDR K. pneumoniae. Hence, antimicrobial stewardship should be implemented as it helps in the prevention of the emergence and the cross-transmission of MDR organisms. Carbapenems should be kept as a last resort drugs. Though this study has shown the trend of tigecycline susceptibility in K. pneumoniae causing neonatal septicemia, the clinical efficacy of tigecycline in neonatal sepsis has not yet been established and clinicians should be more cautious when implementing tigecycline for the treatment of neonatal septicemia.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Change in spectrum of microbial aetiology in relation to gestational age and birth weight and emergence of ESBL in tertiary neonatal intensive care units. Int J Biol Med Res. 2011;2:727-34.
- [Google Scholar]
- Aetiology of community-acquired neonatal sepsis in low and middle income countries. J Glob Health. 2011;1:154-70.
- [Google Scholar]
- Lancet neonatal survival steering team 4 million neonatal deaths: When? Where? Why? Lancet. 2005;365:891-900.
- [Google Scholar]
- The etiology of neonatal sepsis and patterns of antibiotic resistance. J Coll Physicians Surg Pak. 2003;13:449-52.
- [Google Scholar]
- Extended-spectrum beta-lactamase-producing Gram-negative bacteria causing neonatal sepsis in India in rural and urban settings. J Med Microbiol. 2011;60:500-7.
- [Google Scholar]
- National Neonatology Forum NNPD Network (2005). Report of the National Neonatal – Perinatal Database (2002-2003) Available from: http://www.nnfi.org/images/NNPD_2002-03.pdf
- [Google Scholar]
- Tigecycline susceptibility in Klebsiella pneumoniae and Escherichia coli causing neonatal septicaemia (2007-10) and role of an efflux pump in tigecycline non-susceptibility. J Antimicrob Chemother. 2013;68:1036-42.
- [Google Scholar]
- A study of extended spectrum β-lactamase (ESBL) and AmpC β-lactamase producing Klebsiella pneumoniae in neonatal intensive care unit at tertiary care hospital, Ahmadabad. Natl J Community Med. 2012;3:523-8.
- [Google Scholar]
- Carbapenemases mediated resistance among the isolates of neonatal septicemia. J Public Health Med Res. 2013;1:24-7.
- [Google Scholar]
- Tigecycline activity against metallo-ß-lactamase-producing bacteria. Avicenna J Med. 2013;3:92-6.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 17 th Informational Supplement. CLSI document M100-S17. Wayne, PA: Clinical and Laboratory Standards Institute; 2007.
- [Google Scholar]
- Evaluation of methods for AmpC beta-lactamase in gram negative clinical isolates from tertiary care hospitals. Indian J Med Microbiol. 2005;23:120-4.
- [Google Scholar]
- Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-beta-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2003;41:4623-9.
- [Google Scholar]
- Tigecycline susceptibility report from an Indian tertiary care hospital. Indian J Med Res. 2009;129:446-50.
- [Google Scholar]
- CDC, HICPAC. Management of multi-drug resistant organisms in healthcare settings. 2006. :1-4. Available from: http://www.cdc.gov/hicpac/pdf/guidelines/MDROGuideline2006.pdf
- [Google Scholar]
- Neonatal septicaemia by β-lactamases producing multi-resistant organism – cause of concern. 2013. Int J Basic Med Sci. 4:3. Available from: http://www.ijbms.com/micro-biology/neonatalsepticaemia-by-beta-lactamases-producing-multiresistant-organismcause-of-concern-nachhatarjit-singh-pavneet-kaur-aruna-aggarwal/#.UzpaWKiSwvk
- [Google Scholar]
- Neonatal septicemia: Bacteriological profile and antibiogram with special reference to drug resistance. 2014. Int J Recent Sci Res. 5:522-6. Available from: http://www.recentscientific.com
- [Google Scholar]
- Characterization of clinical isolates of Klebsiella pneumoniae from 19 laboratories using the National Committee for Clinical Laboratory Standards extended-spectrum beta-lactamase detection methods. J Clin Microbiol. 2001;39:2864-72.
- [Google Scholar]
- Sepsis in neonates due to imipenem-resistant Klebsiella pneumoniae producing NDM-1 in India. J Antimicrob Chemother. 2011;66:1411-3.
- [Google Scholar]
- Impact of antibiotic resistance in gram-negative bacilli on empirical and definitive antibiotic therapy. Clin Infect Dis. 2008;47(Suppl 1):S14-20.
- [Google Scholar]
- Disk diffusion susceptibility test development for the new glycylcycline, GAR-936. Diagn Microbiol Infect Dis. 1999;35:249-52.
- [Google Scholar]
- High tigecycline resistance in multidrug-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2007;59:772-4.
- [Google Scholar]
- Antimicrobial resistance pattern of multidrug resistant Enterobacteriaceae (MDRE) isolated from clinical samples with special reference to carbapenemase production and susceptibility to tigecycline. Br Microbiol Res J. 2014;4:1035-45.
- [Google Scholar]





