Translate this page into:
Mean Platelet Volume in Type 2 Diabetes Mellitus
Address for correspondence: Dr. Thomas Alex Kodiatte, E-mail: thomaskodiatte@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
diabetes mellitus is a global pandemic. The increased platelet activity may play a role in the development of vascular complications of this metabolic disorder. The mean platelet volume (MPV) is an indicator of the average size and activity of platelets. Larger platelets are younger and exhibit more activity.
Aims:
to determine the MPV in diabetics compared to nondiabetics, to see if there is a difference in MPV between diabetics with and without vascular complications, and to determine the correlation of MPV with fasting blood glucose, glycosylated hemoglobin (HbA1c), body-mass index, and duration of diabetes in the diabetic patients.
Materials and Methods:
platelet counts and MPV were measured in 300 Type 2 diabetic patients and 300 nondiabetic subjects using an automated blood cell counter. The blood glucose levels and HbA1c levels were also measured. Statistical evaluation was performed by SPSS using Student's t test and Pearson correlation tests.
Results:
the mean platelet counts and MPV were higher in diabetics compared to the nondiabetic subjects [277.46 ± 81 X 109/l vs. 269.79 ± 78 X 109/l (P= 0.256)], 8.29 ± 0.74 fl versus 7.47 ± 0.73 fl (P= 0.001), respectively. MPV showed a strong positive correlation with fasting blood glucose, postprandial glucose and HbA1C levels (P=0.001).
Conclusions:
our results showed significantly higher MPV in diabetic patients than in the nondiabetic subjects. This indicates that elevated MPV could be either the cause for or due to the effect of the vascular complications. Hence, platelets may play a role and MPV can be used as a simple parameter to assess the vascular events in diabetes.
Keywords
Diabetes mellitus
hyperglycemia
mean platelet volume
INTRODUCTION
Diabetes mellitus (DM) is a major global health problem.[12] According to estimates of the World Health Organisation, there were 346 million people suffering from diabetes worldwide in 2011.[3]
The increased platelet activity is emphasized to play a role in the development of vascular complications of this metabolic disorder.[4] Platelet volume, a marker of the platelet function and activation, is measured as mean platelet volume (MPV) by hematology analyzers. Diabetic patients have an increased risk of developing micro- and macrovascular disease, and platelets may be involved as a causative agent with respect to altered platelet morphology and function.[56]
The aim of our study was to determine if platelets were activated in diabetes and in its associated vascular complications by measuring the MPV in the diabetics compared to the nondiabetics, to see if there was a difference in MPV in diabetics with and without vascular complications, and to determine the correlation of MPV with fasting blood glucose (FBS), postprandial plasma glucose (PPBS), glycosylated hemoglobin (HbA1c), body-mass index (BMI), and duration of diabetes in the diabetic patients, respectively. We also compared the MPV of diabetics having HbA1c < 6.5% to that of diabetics having HbA1c ≥ 6.5%.
MATERIALS AND METHODS
This was a cross-sectional study carried out in 300 patients who were already diagnosed to have Type 2 DM and 300 nondiabetic subjects without known coronary artery disease. All the diabetic and nondiabetic subjects underwent a complete clinical evaluation with specific reference to any associated macro- or microvascular complications as well as any drugs taken. Height and weight of all the subjects were recorded. We measured the MPV and platelet counts in the above target groups who had a complete blood count done using an automatic blood counter (Beckman Coulter Act5Diff). Venous blood samples were collected in dipotassium EDTA and tested within 1 hour of collection to minimize variations due to sample aging. Samples were maintained at room temperature. Samples for plasma glucose estimation and HbA1c were collected in sodium fluoride and dipotassium EDTA, respectively. The estimation of plasma glucose levels (fasting plasma glucose and postprandial plasma glucose) was carried out by the glucose oxidase method in the auto analyzer (Johnson and Johnson vitros 250) and that of HbA1c by the high-performance liquid chromatography method.
Male patients with hemoglobin below 13 gm% and female patients below 12 gm% were excluded from the study because nutritional anemias can be a cause for reactive thrombocytosis and hence, increased MPV. Nondiabetic subjects with coronary artery disease and diabetics on antiplatelet drugs such as aspirin and clopidogrel were also excluded. Subjects with any diagnosed malignancy were also excluded.
After baseline evaluation, diabetic patients were divided into two groups according to their HbA1c levels: group A consisted of patients with HbA1c levels < 6.5% and group B consisted of patients with HbA1c levels ≥ 6.5%. The latest HbA1c cut-off for diabetic range according to American Diabetic Association 2010 criteria is ≥ 6.5%.
Statistical evaluation was performed by statistical package for the social sciences (SPSS) version 14 (Chicago, IL) for Windows statistics program using Student's independent sample two-tailed t-test and Pearson correlation test (r value as the coefficient). Data were expressed as mean ± standard deviation. A P value <0.05 was considered statistically significant.
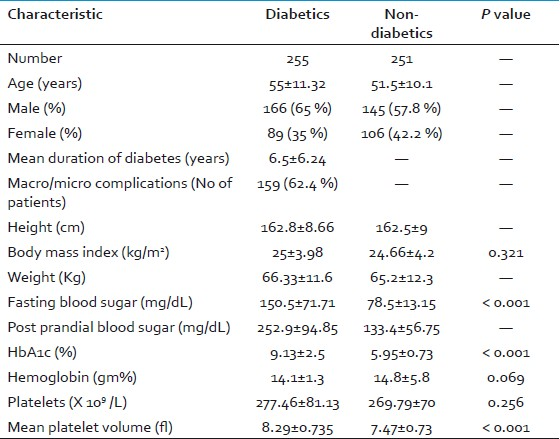
RESULTS
Among the 300 diabetic subjects in the study, 45 were excluded due to anemia or subjects on antiplatelet drugs. Similarly, among the 300 nondiabetic individuals, 49 were also excluded due to anemia or subjects who had history of coronary artery disease. There were 166 male diabetics and 89 female diabetics in the study (255 in total). There were 145 nondiabetic males and 106 nondiabetic females in the study (251 in total). The mean age of the diabetic population was 55±11.32 years, whereas that of nondiabetic population was 51.5±10.1 years. The mean duration of diabetes was 6.5±6.24 years. Out of the 255 diabetics, 159 (62.4 %) had complications such as hypertension, peripheral neuropathy, autonomic neuropathy, diabetic foot, diabetic retinopathy, diabetic nephropathy, coronary artery disease, peripheral vascular disease, hypertriglyceridemia, and hypercholesterolemia and 96 (37.6 %) did not have any of these complications. The mean BMI in the diabetic group was 25±3.98 kg/m2 whereas it was 24.66±4.2 kg/m2 in the nondiabetic group (P=0.321). The mean FBS level in the diabetic population was 150.5±71.7 mg/dL while that of the nondiabetic group was 78.56±13.15 mg/dL (P < 0.001). The mean PPBS level in the diabetic population was 252.9±94.85 mg/dL while that of the nondiabetic group was 133.4±56.75 mg/dL (P < 0.001). The mean HbA1c level in the diabetic group was 9.13±2.53% as compared to 5.95±0.723% of the nondiabetic group (P < 0.001). The mean platelet count in the diabetic group was 277.46±81.13 × 109/L as compared to 269.79±70 × 109/L of the nondiabetic group (P=0.256). In the diabetic subjects, MPV was significantly higher (8.29±0.735 fl) as compared to the non-diabetic group (7.47±0.726 fl; P < 0.001)[Table 1]. Among the diabetic subjects, a positive statistical Pearson correlation was seen between MPV and HbA1c levels (r = 0.29; P < 0.001), FBS levels (r = 0.269; P < 0.001) and PPBS levels (r = 0.194; P = 0.002). However, no statistical correlation was seen between MPV and the duration of DM, BMI and the vascular complications in the diabetic group [Table 2].

In the diabetic group, the mean MPV in subjects with complications (8.35±0.73 fl) were higher than that of subjects without complications (8.2±0.74 fl) but independent student t-test did not show any statistical significance (P = 0.145).
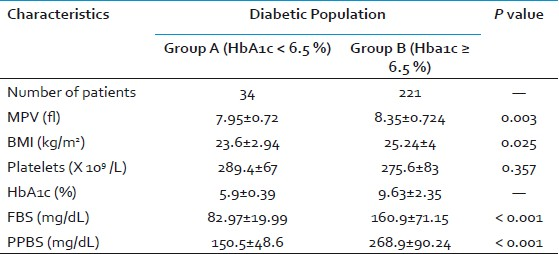
We also divided the diabetic group based on the HbA1c levels into group A (HbA1c < 6.5%) and group B (HbA1c ≥ 6.5%). Out of 255 DM patients, there were 34 patients in group A (mean HbA1c = 5.9±0.39%) and 221 patients in group B (mean HbA1c = 9.63±2.35%). The mean BMI in group A (23.6±2.94 kg/m2) was significantly lower than that of group B (25.24±4 kg/m2; P = 0.025). The mean FBS level in group A was 82.97±19.99 mg/dL while that of group B was 160.9±71.15 mg/dL (P < 0.001). The mean PPBS level in group A was 150.5±48.6 mg/dL while that of group B was 268.9±90.24 mg/dL (P < 0.001). The mean platelet count in group A (289.4±67 × 109/L) was higher than that of group B (275.6±83 × 109/L) but was not statistically significant. The mean MPV in group A (7.95±0.72 fl) was significantly lower than that of group B (8.35±0.724 fl; P = 0.003) [Table 3].
DISCUSSION
DM is a complex metabolic syndrome characterized by chronic hyperglycemia resulting in complications affecting the peripheral nerves, kidneys, eyes, and micro- and macrovascular structures.[4] The prevalence of all types of diagnosed diabetes in most western societies is 3–7%.[12] Countries with the highest absolute number of diabetics are in India (19 million), China (16 million), and the United States (14 million).[12] The prevalence of diabetic microvascular complications is higher in people with poor glycemic control, longer duration of DM, associated hypertension, and obesity.[6] This leads to increased morbidities and mortalities in DM. Diabetes and its vascular complications can cause a financial havoc, become a burden to a country's national economy and dent its growth. India, having the highest number of diabetics, faces such issues. MPV can be used as a simple economical test in the monitoring of DM and thereby help curb the morbidity and mortality.
Preventing vascular complications and monitoring of DM are the need of the hour as the prevalence of DM and its vascular burdens are increasing day by day. Type 2 DM is characterized mainly by impaired insulin secretion and increased tissue insulin resistance.[4] Sustained hyperglycemia leads to a series of interrelated alterations that can cause evident endothelial dysfunction and vascular lesions in diabetic complications.[7] Formation of advanced glycation end products, activation of protein kinase C and disturbances in polyol pathways are the possible mechanisms by which increased glucose induces vascular abnormalities.[8]
Platelets are small discoid blood cells that circulate and participate in hemostasis. Primary plug formation due to platelets seals the vascular defects and provides the required phospholipid surface for the recruited and activated coagulation factors.[9] In response to stimuli generated by the endothelium of blood vessels, platelets change shape, adhere to subendothelial surfaces, secrete the contents of intracellular organelles, and aggregate to form a thrombus.[9] These pro-aggregatory stimuli include thrombin, collagen, epinephrine, ADP (dense storage granules), and thromboxane A2 (activated platelets).[9] Thus, platelets may assume an important role in signaling of the development of advanced atherosclerosis in diabetes.[79–11]
MPV is an indicator of the average size and activity of platelets. Larger platelets are younger, more reactive and aggregable. Hence, they contain denser granules, secrete more serotonin and β-thromboglobulin, and produce more thromboxane A2 than smaller platelets.[511–13] All these can produce a pro-coagulant effect and cause thrombotic vascular complications. This suggests a relationship between the platelet function especially MPV and diabetic vascular complications thus indicating changes in MPV reflect the state of thrombogenesis.[57] There might be small bleeds due to the rupture of atherothrombotic plaques leading to increased platelet recruitment, hyper reactivity, and bone marrow stimulation. High MPV is emerging as a new risk factor for the vascular complications of DM of which atherothrombosis plays a major role.[6] Thus, DM has been considered as a “prothrombotic state” with increased platelet reactivity.[14] Platelet hyperactivity has been reported in diabetics and animals, both in vivo and in vitro.[715]
If vascular damage was only due to increased number of large and reactive platelets, then the rate of damage would have been constant for the duration of disease and independent of diabetic control.[511] This clearly shows that platelet reactivity alone cannot explain the progression of vascular complications in DM since there are other vascular risk factors that may be influenced by the degree of control of diabetes.[511] This was supported by the nonsignificant statistical correlation between MPV and duration of diabetes in the study. A direct relation between platelet dysfunction and the development of diabetic complications has yet to be firmly established.[57]
Platelet hyper-reactivity and increased baseline activation in patients with diabetes is multifactorial.[1116–19] It is associated with biochemical factors such as hyperglycemia and hyperlipidemia, insulin resistance, an inflammatory and oxidant state and also with increased expression of glycoprotein receptors and growth factors.[1116–19] Hyperglycemia can increase platelet reactivity by inducing nonenzymatic glycation of proteins on the surface of the platelet, by the osmotic effect of glucose and activation of protein kinase C.[16–18] Such glycation decreases membrane fluidity and increases the propensity of platelets to activate.[16–18] Platelet function is directly regulated by insulin via a functional insulin receptor (IR) found on human platelets.[16–18] In vivo experiments have confirmed that insulin inhibits platelet interaction with collagen and attenuates the platelet aggregation effect of agonists in healthy nonobese individuals.[16–18] In inflammation, superoxide increases intraplatelet release of calcium after their activation and thus enhancing platelet reactivity.[16–18] Furthermore, superoxide limits the biologic activity of nitric oxide (NO) because the oxidative stress impairs endothelial function that reduces production of NO and prostacyclin.[16–18] Decreasing the effect of NO brings about increased platelet reactivity.[16–18] Platelets from patients with diabetes express more surface P-selectin and glycoprotein (GP) IIb/IIIa receptors and are more sensitive to agonist stimulation than platelets from patients without diabetes.[19] Platelets in DM have dysregulated signaling pathways that lead to an increased activation and aggregation in response to a given stimulus (platelet hyper-reactivity).[1718] Platelet activation contributes to the pathology by triggering thrombus formation and causing microcapillary embolization with the release of constrictive, oxidative, and mitogenic substances such as platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) that accelerate progression of local vascular lesions like the neovascularization of lens in diabetic retinopathies.[18]
At the same time, MPV can also be elevated as an end result of an atherothrombotic event like myocardial infarction. This could be due to the quicker consumption of smaller platelets in the vascular event and compensatory production of reticulated platelets.[2021]
In our study, the mean platelet count in the diabetic group was higher than that of the nondiabetic group that was similar to the studies done by Demirtunc et al.[4] and Zuberi et al.[6] Other studies by Hekimsoy et al.[5] had observed the opposite finding with lower platelet counts in the diabetic group compared with nondiabetic healthy subjects. Hence, the platelet count could be dependent on several variables, that is, mean platelet survival, platelet production rate, and turnover rate in DM.
In our study, the diabetic group had significantly higher MPV than the nondiabetic subjects. This agreed with the findings seen in studies done by Hekimsoy et al., Demirtunc et al., Zuberi et al., Atea et al., Jindal et al., and Papanas et al.[4–6121422]
In our study, higher values of MPV were observed in diabetic subjects with microvascular complications such as retinopathy and microalbuminuria but were not statistically significant. Higher values were also seen in the studies done by Ates et al.[12] and Papanas et al.[22] This suggested a role for the increased platelet activity in the pathogenesis of vascular complications. On the other hand, in the studies done by Hekimsoy et al. and Demirtunc et al. MPV was not significantly different in subjects with diabetic neuropathy/retinopathy from that of diabetics without those complications.[45] Their possible explanation was centered on the rapid consumption of activated platelets in diabetics with complications.[45]
In our study, MPV was significantly higher in diabetics with HbA1c levels ≥ 6.5% than in diabetics with HbA1c levels < 6.5%. There was also a significant association between HbA1c and MPV, which was again seen in the study done by Demirtunc et al.[4] Therefore, it may be concluded that glycemic control decreases the hyper activity of the platelet function and thus may prevent or delay possible diabetic vascular complications. However, our data needs to be further confirmed in larger studies. The reason for a high number of diabetics with HbA1c levels ≥ 6.5% in the current study (221) might have been due to poor dietary practices and lack of knowledge regarding the diet and exercise regimens that ought to be followed in diabetics.
No MPV association was seen with duration of diabetes, BMI and presence of complications. Similar findings were seen in other studies.[45] But our findings were in contrast to the study done by Ates et al.[12] where MPV was positively correlating with the degree of retinopathy in their cases.
The limitations of the study that could not be assessed were qualitative platelet disorders and other reactive causes for raised platelets like menstruation that constitute a minor role.
CONCLUSIONS
Our study showed that in diabetes mellitus, platelets become more reactive and aggregable and their mean volume (MPV) is increased. The increased platelet size may be one factor in the increased risk of atherosclerosis associated with diabetes mellitus and associated vascular complications. Hence, MPV would be a useful prognostic marker of cardio-vascular complications in diabetes. We also found that increase in HbA1c concentration was directly proportional to increased MPV. However, the increased MPV as the cause or the end result of vascular complications needs to be further explored. Hence, we propose that MPV can be used as a simple and cost-effective tool to monitor the progression and control of DM and its cardio-vascular complications.
Source of Support: Nil.
Conflict of Interest: Nil.
REFERENCES
- Hematological Changes in Tobacco using Type 2 Diabetic Patients. Gomal J Med Sci. 2010;8:8-11.
- [Google Scholar]
- Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414-31.
- [Google Scholar]
- World Health Organization August 2011. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/
- [Google Scholar]
- The relationship between glycemic control and platelet activity in type 2 diabetes mellitus. J Diabetes Complications. 2009;23:89-94.
- [Google Scholar]
- Mean platelet volume in Type 2 diabetic patients. J Diabetes Complications. 2004;18:173-6.
- [Google Scholar]
- Comparison of mean platelet volume in patients with diabetes mellitus, impaired fasting glucose and non-diabetic subjects. Singapore Med J. 2008;49:114-6.
- [Google Scholar]
- Platelet activation in patients with diabetic retinopathy. Korean J Ophthalmol. 2003;17:140-4.
- [Google Scholar]
- The Endocrine System. In: Kumar V, Abbas AK, Fausto N, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease (8th ed). New Delhi: Elsevier; 2010. p. :1097-164.
- [Google Scholar]
- Hemodynamic Disorders, Thromboembolic Disease and Shock. In: Kumar V, Abbas AK, Fausto N, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease (8th ed). New Delhi: Elsevier; 2010. p. :111-34.
- [Google Scholar]
- Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54:2430-5.
- [Google Scholar]
- The platelet in diabetes-focus on prevention of ischemic events. Diabetes Care. 2003;26:2181-8.
- [Google Scholar]
- Association of Mean Platelet Volume With The Degree of Retinopathy in Patients with Diabetes Mellitus. Eur J Gen Med. 2009;6:99-102.
- [Google Scholar]
- The Role of Mean Platelet Volume as a Predicting Factor of Asymptomatic Coronary Artery Disease. Korean J Fam Med. 2010;31:600-6.
- [Google Scholar]
- Platelet indices in diabetes mellitus: indicators of diabetic microvascular complications. Hematology. 2011;16:86-9.
- [Google Scholar]
- Factors contributing to increased platelet reactivity in people with diabetes. Diabetes Care. 2009;32:525-7.
- [Google Scholar]
- Platelet function in patients with diabetes mellitus: from a theoretical to a practical perspective. Int J Endocrinol. 2011;2011:742719.
- [Google Scholar]
- Effects of improved metabolic control on platelet reactivity in patients with type 2 diabetes mellitus following coronary angioplasty. Diab Vasc Dis Res. 2006;3:52-6.
- [Google Scholar]
- High mean platelet volume after myocardial infarction: is it due to consumption of small platelets? Br Med J (Clin Res Ed). 1984;289:1576-8.
- [Google Scholar]
- Assessment of mean platelet volume in coronary artery disease- what does it mean? Thromb Res. 2007;120:11-3.
- [Google Scholar]
- Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004;15:475-8.
- [Google Scholar]





