Translate this page into:
Metastasis of renal cell carcinoma to urinary bladder: A rare case report with review of literature
Address for correspondence: Prof. Jain Manoj, Department of Pathology, SGPGIMS, Lucknow - 226 014, Uttar Pradesh, India. E-mail: mjain@sgpgi.ac.in
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Metachronous metastatic renal cell carcinoma (RCC) to bladder is rare incidence. We report a case of RCC with metachronous metastasis to the urinary bladder occurring 2 years postradical nephrectomy. In a follow-up for the past 1 year, the patient is doing well. To the best of our knowledge, this case is the second case of bladder metastasis from RCC in the Indian literature. We reviewed literature and discuss the histopathological features of bladder metastasis of RCC.
Keywords
Metachronous
metastatic renal cell carcinoma
urinary bladder
Introduction
Metastatic renal cell carcinoma (RCC) of urinary bladder is quite uncommon. The prognosis of patients with RCC metastasising to urinary bladder is poor.[1] RCC is the most common renal tumor. Approximately one-third of RCC patients have metastatic disease at the initial diagnosis, and 30% of all patients develop distant metastases later on.[1] Lung (75%), liver (40%), bone (40%), soft tissues (34%), and pleura (31%) are the most common sites of RCC metastasis.[2] However, RCC can metastasize to almost any organ within the body, including thyroid, pancreas, spleen, skin, intestine, heart, and also urinary bladder.[3] The metastatic spread from RCC to urinary tract, especially to bladder is a rare event and is described in <0.3% cases of RCC, and is usually associated with metastasis to other organs.[4] Metastatic RCC accounts for <2% of all bladder tumors with very few cases published in literature.[5] In India, Only one case of RCC with synchronous solitary urinary bladder metastasis in a patient of von Hippel-Lindau have been reported previously, from India.[6] We report a case of RCC with solitary metachronous metastasis to the urinary bladder occurring 2 years after radical nephrectomy. To the best of our knowledge, this is the second case reported from India of solitary RCC metastasis to urinary bladder without evidence of metastasis to any other site.
Case Report
A 55-year-old male patient presented to the urology outpatient department with chief complaints of right-sided flank pain radiating to right inguinal region. He was not diabetic, hypertensive, hypothyroid, or hyperlipidemic. Ultrasonographic study done outside showed a homogeneous hypoechoeic well-defined mass at lower pole of right kidney. CECT revealed a heterogeneously enhancing lobulated mass of size 7.9 cm × 6.6 cm × 7.8 cm arising from lower pole of right kidney with areas of necrosis. The lesion was localised within renal fascia with no extension to adjacent structures. Right renal vein and IVC showed normal luminal contrast opacification. Left kidney showed a cortical cyst at upper pole measuring 5.7 cm × 4.4 cm. There was no lymphadenopathy. The patient underwent laparoscopic right radical nephrectomy. Grossly the right radical nephrectomy specimen measured 18 cm × 7.5 cm × 6 cm. Cut section showed a circumscribed solid gray-brown tumor in the lower pole of kidney measuring 7 cm x 6.5 cm x 5.5 cm with foci of hemorrhage and necrosis [Figure 1]. Microscopic examination revealed a well-encapsulated tumor composed of cells arranged in sheets and nests separated by fibrovascular septa. The cells were polygonal with prominent cell membrane, abundant clear cytoplasm, round nuclei, dispersed chromatin, and prominent nucleoli. Large areas of ischemic necrosis were seen. The tumor was confined within the capsule. Renal pyramids, sinus, perinephric fat vascular and ureteric resection margin were free. Immunohistochemically tumor cells were positive for CD10, vimentin, and AE1/AE3. Based on these findings, the case was reported as Clear cell RCC (WHO/ISUP Grade 3) [Figure 2].

- Gross appearance of tumor arising from the lower pole of kidney: A well-circumscribed mass with areas of necrosis and focal hemorrhages
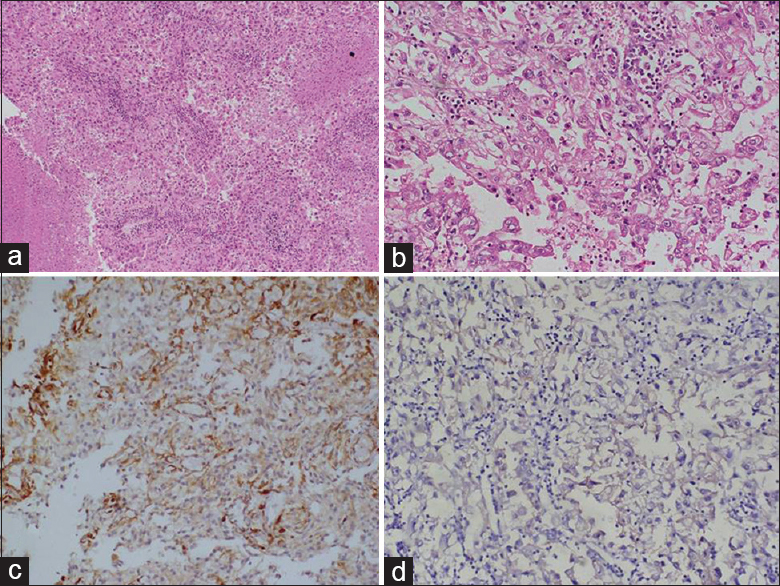
- (a) Microscopic appearance of the tumor composed of round cells with amphophilic to clear cytoplasm, area of necrosis is also seen (low left) (H and E, ×100), (b) Higher magnification shows cells with well-defined cell border, abundant clear cytoplasm, irregular nuclear profile with prominent nucleoli. (H and E, ×200), (c) Tumor cells are positive for vimentin, blood vessel wall act as internal control (IPX, ×100), (d) Tumor cells show moderate membranous positivity for CD10, (IPX, ×100)
The postoperative course was uneventful, and patient was on regular follow-up every 3 months.
After 2 years of nephrectomy the patient had painless hematuria with passage of blood clot. CECT showed 2.2 cm × 2.8 cm focal hypodense polypoidal lesion at anterior wall of urinary bladder. Right kidney was not visualized (postnephrectomy status). Left kidney showed cortical cyst at upper pole as was seen in previous scan. Further radiological evaluation revealed negative studies for metastatic disease at other sites, including a chest X-ray with lung tomography and radioisotopic bone scans. Cystoscopy showed a 3 cm × 4 cm solitary solid pedunculated growth at anterior wall and adjoining dome. Rest of the urinary bladder mucosa was unremarkable. The mass was completely removed transurethrally without any massive intraoperative hemorrhage. Histopathological examination of the resected tumor showed a tumor composed of clear cells with well-defined cell border. The tumor had nested growth pattern with intervening vascular septae. Tumor was confined to lamina propria of the urinary bladder and had striking resemblance to the primary tumor in kidney. Immunohistochemically, the tumor cells were positive for AE1/AE3, vimentin, and CD 10. CK7, CK20, and EMA negativity ruled out clear cell urothelial carcinoma and clear cell adenocarcinoma [Figure 3]. Based on these a diagnosis of metastatic RCC of urinary bladder was made. No evidence of any other organ metastasis in the body was found.
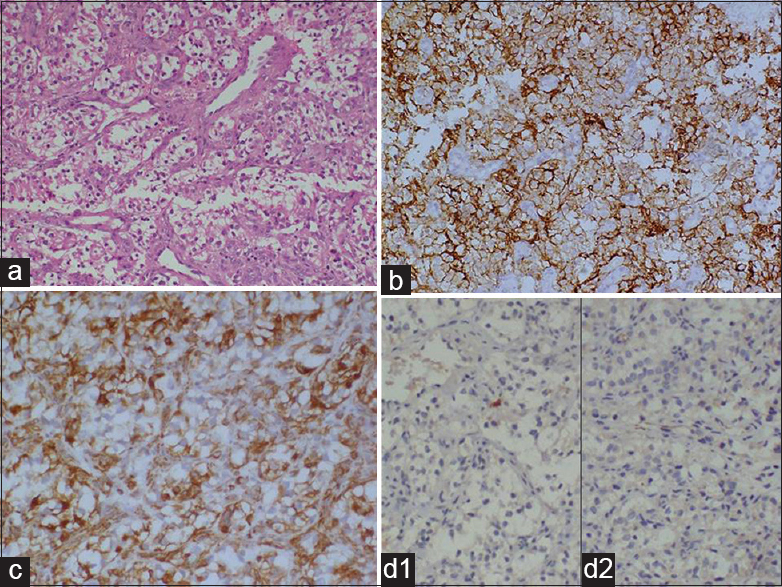
- (a) Microscopic appearance of the tumor from bladder show nested growth of round to polygonal cells with amphophilic to clear cytoplasm and irregular nuclei (H and E, ×100). (b) Neoplastic cells show strong cytoplasmic positivity for CD10 (IPX, ×100), (c) Cytoplasmic vimentin positivity is strong in tumor cells (IPX, ×100), (d1) Tumor cells are negative for CK7 and (d2) CK20 (IPX, ×100)
Discussion
Metastasis of RCC to the urinary tract is very rare. The first case of RCC metastases to the bladder was described by Hoffmain in 1907.[7] It seems to be associated with an advanced tumor stage and poor prognosis with a reduced overall survival as described by several reports.[8] Such metastasis can be synchronous or metachronous.[9] Patients usually have associated widespread tumor dissemination with metastasis to other organs.[10] Even after thorough search there was no evidence of metastasis to any organs other than urinary bladder, in our case.
The pathologic mechanism underlying spread of RCC to urinary bladder remains unknown. Several possible mechanisms have been proposed, including hematogenous metastasis through the general circulation, retrograde spread of the tumor from the renal vein or renal hilar lymphatics down the periureteral veins or lymphatics that connect with pelvic organs, and direct intraluminal transit of tumor cells with seeding of the distal urothelium.[911] RCC commonly metastasizes through the bloodstream, leading to the synchronous discovery of a widespread area of metastasis. In contrast, urinary spread can be suspected if the primary tumor invades into the renal pelvis or at least into the collecting duct.[12] Although in our case, neither there was evidence of hematogeneous spread to other organs nor the primary tumor invaded the renal pelvis, and the pathogenesis remains elusive.
Treatment options for metastatic RCC are limited owing to poor treatment response to chemotherapy and radiation therapy. Prognosis has been reported to be good when only a single metastasis exists in the bladder, and follow-up without additional systemic treatment is possible after the surgical removal of the metastatic lesion in the bladder.[13] In addition, when carcinoma has metastasized to other organs at the time when RCC metastasis is found in the bladder, additional systemic treatment such as immune therapy is required. Since our patient had solitary bladder metastasis, transurethral resection of bladder tumor was performed for complete surgical resection of metastasis tumor. The patient has no evidence of bladder recurrence after the complete removal of the bladder mass in a 1 year follow-up.
Histologically, metastatic RCC of urinary bladder has to be differentiated from clear cell adenocarcinoma of bladder which shows a predilection for affecting the female urethra. These adenocarcinomas have tubular, cystic, papillary, or diffuse growth pattern. The tumor cells lining the tubules and cysts may be cuboidal, hobnail, or flattened and usually show strong immunostaining for PAX 8. Lipoid-cell variant of urothelial carcinoma is another differential diagnosis that is to be considered. The tumor is composed of pleomorphic cells with high-grade nuclear atypia and large, intracytoplasmic vacuoles imparting an adipocytic morphology. These intracellular vacuoles are negative for mucin stain, alcian blue, and periodic acid–Schiff stains, reasonably pointing toward lipid. Clear cell carcinomas arising from other organs such as prostate, lung, breast, uterus, ovary, and vagina may rarely metastasize to bladder. Other possibility including metastatic melanoma, clear cell sarcoma, and seminoma need to be considered.[14] In the present case, based on the morphology and immunohistochemistry the above-mentioned differential were ruled out.
Conclusion
RCC metastasis to the bladder is extremely rare, and may present either synchronously or metachronously. This possibility should be kept in mind in patients of RCC having hematuria postnephrectomy. From a Pathologist perspective, this differential, though rare, should always considered in the differential of clear cell tumors of urinary bladder in any patients with or without antecedent history of RCC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Natural history and therapy of metastatic renal cell carcinoma: The role of interleukin-2. Cancer. 1997;80:1198-220.
- [Google Scholar]
- Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004.
- Renal cell carcinoma: Unusual systemic manifestations. Medicine (Baltimore). 1976;55:291-311.
- [Google Scholar]
- Metastasis to ureters and urinary bladder from renal carcinoma; Report of two cases. J Int Coll Surg. 1956;25:117-26.
- [Google Scholar]
- An unusual case of von hipple lindau (VHL) syndrome with bilateral multicentric renal cell carcinoma with synchronous solitary urinary bladder metastasis. Int Urol Nephrol. 2007;39:11-4.
- [Google Scholar]
- Ureteral tumor thrombus from renal cell carcinoma extending into bladder. Urol Oncol. 2007;25:393-5.
- [Google Scholar]
- Metastatic renal cell carcinoma to the bladder: A clinicopathologic and immunohistochemical study. Mod Pathol. 1999;12:351-5.
- [Google Scholar]
- Long-term survival after “drop metastases” of renal cell carcinoma to the bladder. Urology. 2002;60:697.
- [Google Scholar]
- Synchronous metastatic renal cell carcinoma to the genitourinary tract: Two rare case reports and a review of the literature. Can J Urol. 2009;16:4611-4.
- [Google Scholar]
- A solitary and synchronous metastasis of renal cell carcinoma to the bladder. Int Urol Nephrol. 1994;26:529-33.
- [Google Scholar]
- Clear cell urothelial carcinoma of the urinary bladder: A case report and review of the literature. J Med Case Rep. 2014;8:275.
- [Google Scholar]





