Translate this page into:
Need for appropriate specimen for microbiology diagnosis of chronic osteomyelitis
Address for correspondence: Dr. Sukanya Sudhaharan, Department of Microbiology, Nizam's Institute of Medical Sciences, Panjagutta, Hyderabad - 500 082, Telangana, India. E-mail: sukanyavimala@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
INTRODUCTION:
Chronic osteomyelitis (COM) is a common infection, especially in developing countries. An adequate bone biopsy specimen processed with appropriate microbiology culture methods for isolation and identification of the causative organisms is considered as the gold standard for the diagnosis of osteomyelitis.
MATERIALS AND METHODS:
The present study is a retrospective microbiology analysis of the specimen from 219 clinically diagnosed cases of COM between January 2013 and April 2016.
RESULTS:
The overall culture positivity was 111/219 (50. 6%), colonization was seen in 22/219 (10.5%), while the rest 86/219 (39.3%) were culture-negative specimen; culture positivity was highest from tissue specimen (71/113, 62.8%). Among the swabs, 40/106 (37.7%) were culture positive. About 28/40 (70%) culture-positive swabs showed significant growth of Gram-positive organisms. Colonization with skin flora such as diphtheroids and Coagulase-negative Staphylococci was seen in 22/106 (20.7%) of the swabs. Sterile cultures (44/106, 41.6%) were high among the swab specimen. Gram-positives were most common (75/111, 67.56%). Staphylococcus aureus was the predominant organism isolated in 70/111 (63%) cases. Gram-negative bacilli showed a high level of antibiotic resistance.
CONCLUSION:
As per our data, the culture yield from wound swabs was low or contaminated with normal skin flora, as compared to the biopsy or tissue specimen. Hence, an appropriate sampling of the infected bone using recommended protocols is highly essential for improving microbiological yield and the outcome of COM.
Keywords
Antibiotic resistance
bone biopsy
Staphylococcus aureus
tissue
Introduction
Chronic osteomyelitis (COM) was first described in the Hippocrates era.[1] Although the incidence of osteomyelitis has reduced to a certain extent with the advent of antibiotics and chemotherapeutic agents, it continues to be one of the most challenging, especially in developing countries.[2] Osteomyelitis can occur at any age and can involve any bone. The infection results from hematogenous seeding, contiguous spread, or direct inoculation of microorganism(s) into intact bone as occurs with violent trauma, bone surgery, or joint replacement surgery.[34] The infection can be limited to a single portion of the bone or can involve several regions, such as marrow, cortex, periosteum, and the surrounding soft tissue.[456] Progressive bony destruction associated with avascular necrosis of bone and formations of sequestrum (dead bone) are hallmarks of osteomyelitis.[6] Prognosis of COM depends heavily on proper identification and treatment of the bone-infecting organism(s) with appropriate antibiotics. Isolation of the causative organism(s) can be achieved by direct biopsy of the involved bone and is considered the gold standard for conclusive microbiological diagnosis.
The present study is a retrospective microbiology analysis of the specimen from clinically diagnosed cases of COM at a tertiary care hospital in south India. The purpose of this analysis is to emphasize the importance and need to obtain an appropriate specimen for the microbiological diagnosis of COM.
Materials and Methods
Data, retrieved from the microbiology records of specimen submitted from patients, with a confirmed diagnosis of COM and managed at our hospital between January 2013 and April 2016, were analyzed. All the patients’ records were subsequently screened for gender, age, underlying cause for the infection of the bone, bone involved, surgical access to obtain the bone specimen, and source of specimens submitted for culture.
Microbiological processing
Specimen submitted for microbiology processing included debrided infected bone or any purulent nonbone specimens directly related to the infected bone such as pus aspirated from surrounding soft tissues and drainage from sinus tracts collected as swabs. The specimens were processed using standard microbiological techniques (CMPH).[7] All the tissue specimens were processed simultaneously for mycobacteria using liquid (Bact/ALERT 3D) (bioMerieux, Marcy l’Etoile, France) and solid cultures (LJ medium). The latter was incubated for 6 weeks at 37°C. Gram's stain was done on all specimens. The primary cultures were performed on 5% sheep blood agar (COS) and chromogenic agar (CPS) (bioMerieux, Marcy l’Etoile, France) and inoculated into in-house prepared liquid thioglycollate (tissues/bone and purulent materials) or into Trypticase soy broth (swabs) and incubated at 37°C for 48 h. All cultures were examined at 24 and 48 h. Identification and antimicrobial susceptibility pattern of the bacterial isolates were done using the Vitek 2 (bioMerieux, Marcy l’Etoile, France) system. Any thioglycollate broth that was turbid was subcultured on 5% sheep blood agar and incubated anaerobically in an anaerobic pouch with Anaerobic gas pack (bioMerieux, Marcy l’Etoile, France). The pouch was opened and plates were inspected for anaerobic organisms. In case of the swabs, all turbid Trypticase soy broths were subcultured on COS and CPS. A swab specimen was considered to be colonized when there was scanty growth <100 CFU or when the culture plates were sterile while the Trypticase soy broth showed growth on subculture. Only the first positive culture from each identified patient was included in this analysis.
Results
In all, 219 patients with COM were documented during the study period. There was a male preponderance (189/219, 86.3%) with majority in the age group of 10–20 years. Trauma was the major risk factor for osteomyelitis (113/219, 51.5%). The lower limb bones were more commonly affected of which femur (197/219, 89.9%) was the predominant bone involved.
Table 1 shows the specimen-wise isolation of the various organisms. In all, 102/219 (46.5%) tissue specimens or purulent aspirates obtained through a surgical procedure and 106/219 (48.4%) swabs collected from open and draining sinuses and 11 (5.5%) paired specimens (tissue/pus and swab) were submitted for microbiological processing. (These paired specimens are considered as tissue for analysis purpose).
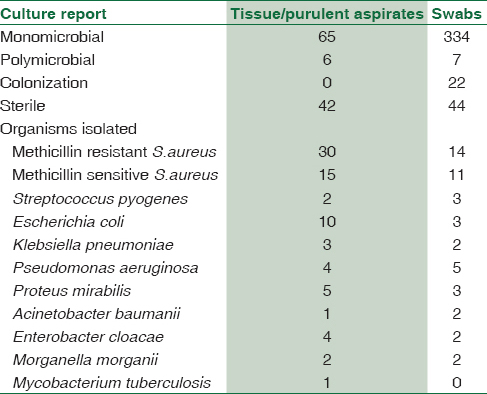
The overall culture positivity was 111/219 (50.6%), colonization was seen in 22/219 (10.5%), while the rest 86/219 (39.3%) were culture-negative specimen.
Among the culture-positive specimen, 98/111 (88.2%) had monomicrobial growth, while polymicrobial growth was seen in 13/111 (11.7%).
Culture positivity was highest from tissue specimens (62/102, 60.7%) while 9/11 (81.2%) of the paired specimens were culture positive and overall 71/113 (62.8%) of the tissues were culture positive. Among the swabs, 40/106 (37.7%) were culture positive. About 28/40 (70%) culture-positive swabs showed significant growth of Gram-positive organisms. Colonization with skin flora such as diphtheroids and Coagulase-negative Staphylococci was seen in 22/106 (20.7%) of the swabs. Sterile cultures (44/106, 41.6%) were high among the swab specimen.
Among the bacterial isolates, Gram-positives were most common (75/111, 67.5%). Staphylococcus aureus (S. aureus) was the predominant organism isolated in 70/111 (63%) cases. Of these, methicillin-resistant S. aureus was isolated in 44/111 (39.6%) cases. Among Gram-negative bacilli (GNB), Escherichia coli was the most common organism isolated, 13/111 (11.7%) cases. The other organisms isolated included Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii, Proteus mirabilis, Enterobacter cloacae, and Morganella morganii and showed a high level of antibiotic resistance. One patient had a mixed infection with Mycobacterium tuberculosis and A. baumannii (the latter was considered as a secondary pathogen). In our study, neither anaerobic organisms nor any fungi were isolated.
Conservative management with wound care and antibiotic was done for 102/219 (46.5%) while the rest of the 117/219 (53.5%) underwent a relevant surgical procedure to remove the nidus of infection. No mortality was recorded.
Discussion
COM is a relapsing and persistent infection that still remains a major medical problem in most countries. It is a very expensive disease for the patient and society because of the involved costs of diagnosis, inpatient and outpatient treatment, rehabilitation, lost productivity, and the sequel.[3]
The most common predisposing factor of COM in our study was trauma.[8] Violent trauma (primarily open fractures and severe soft-tissue injury), prosthetic surgery on bones and joints, and vascular insufficiency due to underlying morbid conditions, such as diabetes, are the leading causes for COM.
The establishment of COM depends on the size of the inoculum, virulence of the bacteria, and loss of resistance of the host tissues caused by their disruption. The process evolves over months to years and is characterized by low-grade inflammation, presence of dead bone (sequestrum), new bone apposition, and fistulous tracts.[8]
Trauma results in hemorrhage, loss of the intact epithelium, and cell destruction in the region of epiphyseal cartilage followed by diminished tissue resistance. The underlying bone is thus predisposed to infection by the microorganisms either exogenously seeded or a contiguous contaminated source or from the surrounding skin. Metastatic source of infection may be involved as well, for example, percutaneous sutures, suction drains, intravenous catheters, and indwelling urinary catheters.[9] Trauma was the major cause in 51.5% of our cases.
The contaminating organisms begin to multiply and colonize in the metaphysis of the bone and express adhesion factors for the bone matrix, invade the cortical bone, and freely spread within the bone by vascular Haversian canals within the osseous structure. Very soon, the medullary bone and marrow are affected and hasten the spread of pathogens to nonlocalized areas.[41011] At the infarction edge, there is reactive hyperemia associated with increased osteoclastic activity. Subsequently, there is a loss of the bone, localized osteoporosis and exuberant periosteal apposition, triggering the proliferation of osteoblasts and new bone formation in a haphazard fashion. Pockets of bone necrosis (sequestra) ensue, which are devoid of blood supply and can continue to harbor bacteria despite antibiotic treatment.
Diagnosis of COM depends on proper isolation, identification, and treatment of the bone-infecting, often multidrug-resistant (MDR) organism(s). Complications can be further reduced with surgical debridement, removal of the dead tissues, and appropriate antibiotics.
Hence, an adequate bone biopsy specimen with appropriate microbiological cultures of the tissue is considered as the gold standard for the diagnosis and management of COM. The specimen, especially tissue/bone, should be processed both for aerobic and anaerobic cultures and for mycobacterial and fungal pathogens if the clinical features warrant[5] as was done in our laboratory.
Although there are reports that consecutive deep sinus tract specimen predicts the pathogen of osteomyelitis,[12] these specimens should not replace bone biopsy.[10] Ideally, a percutaneous bone biopsy, performed under fluoroscopic guidance, should be followed, avoiding collection through an ulcer or sinus tract, so as to minimize contamination by the colonizing flora. In implant-associated infections, it is recommended to obtain deep specimen from up to five sites around the implant at debridement to optimize diagnostic yield.
As per our analysis, 113 tissue/purulent specimens were collected appropriately and adequately through an invasive procedure, of which 62.8% were culture positive. It has been stated that antibiotics, released slowly from the bone, may affect the culture yield and are then falsely reported as culture negative.[12] The high number of culture-negative specimens (39.3%) in our study was probably due to prior antibiotic therapy. It is recommended that in a stable patient, any prior antibiotic therapy should be stopped at least 1–2 weeks before specimen collection and no routine surgical prophylaxis be given until bone biopsy is performed.
However, ideal procedures of specimen collection may not always be possible to follow and generally an inappropriate and sometimes inadequate material from an open sinus tract or draining ulcer is submitted for microbiology tests which gives misleading results. Such superficial specimens grow only skin flora and nonpathogenic microorganisms colonizing the site and frequently miss the primary pathogen(s).[10] As per our data, only 37.7% of the superficial swabs collected were culture positive while colonization was seen in 20.7%, and 41.6% were sterile, indicating that dry swabs or inadequately collected specimen was probably submitted which jeopardized the culture yield.
The spectrum of pathogens causing COM is based on the associated cause. Monomicrobial infection was seen in 88.2% and polymicrobial growth in 11.7%.[8] Posttraumatic osteomyelitis with bacteria colonization during trauma is an increasing clinical problem.[13] Polymicrobial colonization and thereby infection of the soft tissues are common in patients with posttraumatic COM. The main problem associated with COM is the ability of the microorganisms to remain in necrotic bone tissue for long periods that has not undergone adequate surgical debridement.[14] Furthermore, the bone contamination maybe facilitated by concomitant manipulation of colonized soft tissues performed while reducing the fracture.
S. aureus was the most common pathogen isolated from 70/111 (63%) specimens (42 tissues and 28 swabs). It has been proven by other studies that S. aureus isolated from superficial specimen or a sinus tract is often correlated presumptively to its presence in deep cultures.[15] S. aureus possesses a variety of virulence factors that contribute to the development and chronicity of osteomyelitis. These organisms express proteins called adhesin that facilitate their attachment to the bone and are usually incorporated into a relatively impermeable glycocalyx biofilm, a slime layer, which shields the bacteria from antimicrobial agents. The organisms are internalized by the osteoblasts and survive intracellularly (sometimes in a metabolically altered state in which they appear as so-called small colony variants) resulting in persistence of bone infections.[15] At this point, surgical removal of the nidus of infection is usually necessary for complete resolution of infection.[11]
Isolation of other common pathogens from superficial sites or sinus tracts must be confirmed with a bone culture[6] as even a heavy growth of a common pathogen is suggestive but not diagnostic of its involvement in COM.
From 41/111 culture-positive specimens (30 tissues and 11 swabs), resistant GNB were grown. The importance of these MDR GNB causing COM has increased recently, consequent to the increasing use of prosthetic implants and especially the rising number of high-energy traumas associated with open fractures, as a consequence of traffic accidents.[1] Anaerobes may also play a significant role in producing a resistant COM that does not yield to the normal treatment measures. Therefore, routine culture for anaerobes in osteomyelitis is advocated.[2] Although anaerobic cultures were done, there were no anaerobes isolated in our series.
In one case, M. tuberculosis was isolated from the purulent aspirate. It has been shown that isolated involvement of bone by tuberculous infection is uncommon, and the variable clinical and radiological pictures may mimic chronic pyogenic osteomyelitis.[16]
Conclusion
As evidenced by our data, an appropriate sampling of the infected bone using recommended protocols is highly essential for improving microbiological yield and the outcome of COM. The biopsy or purulent specimen processed with standard microbiology culture methods for isolation and identification of the causative organisms is considered as the gold standard for the diagnosis of osteomyelitis. The increasing rate of MDR GNBs causing COM can be controlled with good principles of antibiotic therapy and aseptic methods of wound care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Gram-negative osteomyelitis: Clinical and microbiological profile. Braz J Infect Dis. 2012;16:63-7.
- [Google Scholar]
- Clinical and microbiological evaluation of osteomyelitis. Bahrain Med Bull. 2001;23:61-5.
- [Google Scholar]
- Lack of microbiological concordance between bone and non-bone specimens in chronic osteomyelitis: An observational study. BMC Infect Dis. 2002;2:8.
- [Google Scholar]
- Duration of post-surgical antibiotics in chronic osteomyelitis: Empiric or evidence-based? Int J Infect Dis. 2010;14:e752-8.
- [Google Scholar]
- Diagnostic value of sinus-tract cultures in chronic osteomyelitis. JAMA. 1978;239:2772-5.
- [Google Scholar]
- Wound cultures. In: Garcia LS, ed. Clinical Microbiology Procedures Handbook Vol 1. (3rd). Washington, DC: ASM Press; 2010. p. :3.13.1.1-2.1.
- [Google Scholar]
- Chronic osteomyelitis: Aetiology and antibiotic susceptibility pattern. Int J Recent Trends Sci Technol. 2014;9:337-40.
- [Google Scholar]
- Bacteriological profile of osteomyelitis with special reference to Staphylococcus aureus. Indian J Pract Doct. 2008;4:1-2.
- [Google Scholar]
- Osteomyelitis and lower extremity amputations in the diabetic population. J Diabetic Foot Complications. 2010;2:18-27.
- [Google Scholar]
- Osteomyelitis: Clinical overview and mechanisms of infection persistence. Clin Microbiol Newsl. 2006;28:65-72.
- [Google Scholar]
- Two consecutive deep sinus tract cultures predict the pathogen of osteomyelitis. Int J Infect Dis. 2010;14:e390-3.
- [Google Scholar]
- Posttraumatic and postoperative osteomyelitis: Surgical revision strategy with persisting fistula. Arch Orthop Trauma Surg. 2014;134:159-65.
- [Google Scholar]
- Recommendations for the treatment of osteomyelitis. Braz J Infect Dis. 2014;18:526-34.
- [Google Scholar]
- Culture of percutaneous bone biopsy specimens for diagnosis of diabetic foot osteomyelitis: Concordance with ulcer swab cultures. Clin Infect Dis. 2006;42:57-62.
- [Google Scholar]





