Translate this page into:
Performance of a 27 kDa Fasciola hepatica Antigen in the Diagnosis of Human Fascioliasis
Address for correspondence: Prof. Bahador Sarkari, E-mail: sarkarib@sums.ac.ir
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Serological diagnosis, based on antigenic fractions of the parasite can be used for the early diagnosis of human fascioliasis. The current study aimed to evaluate the efficacy of a 27 kDa immunodominant antigen of Fasciola hepatica adult worms, in an indirect enzyme-linked immunosorbent assay (ELISA) system for serological diagnosis of human fascioliasis.
Materials and Methods:
The immunodiagnosis of human fascioliasis, using a 27 kDa immunodominant antigen, purified from F. hepatica somatic antigens (SAs), was evaluated by Western blotting and ELISA with sera samples of human fascioliasis patients, healthy controls and patients with other parasitic infections.
Results:
Using western blotting, from 12 sera of fascioliasis patients, 11 sera (91.6%) detected the 27 kDa subunit. None of 30 samples from healthy controls or 32 sera from nonfascioliasis patients reacted with the 27 kDa antigen. Accordingly, sensitivity and specificity of the system was found to be 91.6% and 100%, respectively. The 27 kDa antigen was purified from the SAs and was used in an indirect ELISA system. Of 15 sera of fascioliasis patients, all (100%) were found to be positive by ELISA whereas only 4 cases (6.25%) of nonfascioliasis patients or healthy controls were false-positive by this system. Accordingly, the sensitivity and specificity of the test were 100% and 93.6%, respectively.
Conclusion:
Findings of this study demonstrated that both Western blotting and the indirect ELISA, based on the 27 kDa subunit of F. hepatica SA, are reliable methods for serodiagnosis of human fascioliasis.
Keywords
Enzyme-linked immunosorbent assay
fascioliasis
immunodiagnosis
INTRODUCTION
Human fascioliasis is an emerging/re-emerging helminthic zoonotic disease with the widest known distribution that has partly been related to climate changes.[1] Human become accidentally infected through ingesting aquatic plants or by drinking water contaminated with metacercaria. In an estimate of the disease prevalence, approximately 17 million people are infected with Fasciola spp. around the world.[1] Laboratory tests are the conventional methods for diagnosis of human fascioliasis. Along with laboratory methods, findings of ultrasonographic, computed tomographic, and magnetic resonance imaging as well as clinical findings are helpful for diagnosis of human fascioliasis.[2]
Laboratory tests based on the stool studies for egg of the fluke are not reliable during the prepatent period since the parasite cannot produce an egg before invasion of the biliary ducts. Moreover, eggs might not be present in the stool even after multiple stool examinations due to low number of them and their irregular intervals discharge. Therefore, negative stool examinations do not rule out the fascioliasis. Serological diagnosis, based on antigenic fractions of the parasite can be used for the early diagnosis of the infection.[3] Among the serological methods, the determination of anti-Fasciola antibodies by ELISA is considered a reliable approach for the diagnosis of the infection.[4] The use of specific antigens, purified from somatic or excretory-secretory products of Fasciola spp., in ELISA or immunoblotting system, yielded sensitivity of 92–100% and specificity of 84–100%, as reported in different studies.[56789] Among the subunit antigens, the cathepsin-L, a component of Fasciola excretory/secretary antigens, has received the most attention.[57] However, the performance of such methods are still unsatisfactory and development of diagnostic test, using different subunit antigens of Fasciola is needed. The present study was conducted to evaluate the efficacy of a 27 kDa immunodominant antigen of Fasciola hepatica adult worms, isolated by a simple elution method, in an indirect ELISA system for immunodiagnosis of human fascioliasis.
MATERIALS AND METHODS
Human sera
Serum samples were obtained from 15 fascioliasis patients (five of samples were kindly provided by Prof. Rokni from School of Public Health at Tehran University of Medical Sciences and the rest were obtained from patients from a newly emerging focus of human fascioliasis in Kohgiluyeh and Boyer-Ahmad province, Southwest of Iran). Positive sample, from fascioliasis patients, were selected based on positive parasitological tests (finding ova in stool samples) along with clinical signs and also their positive response to the chemotherapy. Informed consent for participation in the study was obtained from the subjects. Negative samples were taken from healthy individuals from nonendemic area who had no history of fascioliasis. Moreover, thirty two sera were obtained from nonfascioliasis patients including those with hydatidosis (n = 4), hymenolepiasis (n = 5), ascariasis (n = 1), malaria (n = 7), trichostrongyliasis (n = 1), strongyloidiasis (n = 1), taeniasis (n = 1), fever of unknown origin (n = 2), cancer (n = 1) cryptosporidiasis (n = 3), and toxocariasis (n = 6).
Preparation of Fasciola somatic antigen
Adult F. hepatica worms were obtained from infected sheep collected from local abattoirs in Yasuj, Southwest of Iran, and was washed three times with phosphate-buffered saline (PBS) (pH 7.2). Molecular technique (polymerase chain reaction-restriction fragment length polymorphism) was used to find out the species of the Fasciola.[10] Somatic antigen (SA) was prepared by homogenizing the worms in PBS, followed by sonication and centrifugation at 15,000 g at 4°C for 30 min. The supernatant was collected as SA. The protein content of sample was estimated, using Bradford method, and the antigen was kept at -20°C until further use.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by Western blot analysis was used for the detection of immunodominant antigen from the Fasciola SA as essentially described before.[11] The crude SA was subjected to discontinuous reducing SDS-PAGE electrophoresis. A polyacrylamide gel consisted of an upper 5% stacking and a lower 15% separating gel was prepared. After electrophoresis, proteins were electro-transferred onto nitrocellulose membrane. The membranes with blotted antigen were cut into strips and blocked with 5% (w/v) of skimmed milk in washing buffer (PBS containing 0.05% Tween 20) and incubated with sera (at 1:100 dilution) of either fascioliasis patient, or healthy and nonfascioliasis patients. The strips were incubated with horseradish peroxidase conjugated anti-human immunoglobulin, at 1:3000 dilution, (Sigma) and the bound antigens was developed using diaminobanzidine substrate.
Isolation of immunodominant antigen from the polyacrylamide gels
Elution of immunodominant antigen from the polyacrylamide gel was carried out as previously described for purifying proteins from polyacrylamide gels.[12] Protein content of the eluted antigen was measured, using Bradford protein assay.
Enzyme linked immunosorbent assay
Enzyme linked immunosorbent assay (ELISA), using purified immunodominant antigen of F. hepatica SA, was carried out in flat bottom 96-well microplates (Nagle Nunc International, Roskilde, Denmark) as described by Sarkari et al.[13] Briefly, the microplates were coated with 5 μg/ml of purified antigen (100 μl/well) in coating buffer (0.05 M carbonate-bicarbonate buffer, pH 9.6) and incubated at 4°C overnight. Plates were washed 5 times in phosphate-buffered saline-Tween 20 ([PBST], pH 7.4 containing 0.05% Tween 20) and blocked with 3% skimmed milk in PBST for 2 h. Plates were washed as before and 100 μl of serum samples (1/50 and 1/100 dilution in PBST) of fascioliasis patients, nonfascioliasis patients, and healthy controls were added to the plate and incubated for 1.5 h at room temperature. After washing, horseradish peroxidase conjugated polyclonal antibody against human immunoglobulin (Sigma) in PBST (1/2000 dilution) was added to the plates and incubated for 1-h at room temperature. After washing as before, the plates were incubated with OPD substrate. The absorbance at 490 nm was measured, using a microplate reader (BioTek Instruments, Inc.), after 30 min. The cut-off point was set at 3SD above the mean of negative controls.
RESULTS
Using Western blotting with the SAs of F. hepatica, proteins of various molecular weights (from 10 to 175 kDa) reacted with the sera of fascioliasis patients [Figure 1]. From 12 sera of fascioliasis patients, which were tested by Western blotting, 11 sera (91.6%) detected the 27 kDa subunit [10 of the samples are depicted in Figure 1]. None of 30 serum samples from healthy controls or from nonfascioliasis patients (at a dilution of 1:100) reacted with the 27 kDa antigen. Accordingly, sensitivity and specificity of the system was found to be 91.6% (95% confidence interval [CI]: 59–99%) and 100% (95% CI: 93–100%) respectively.
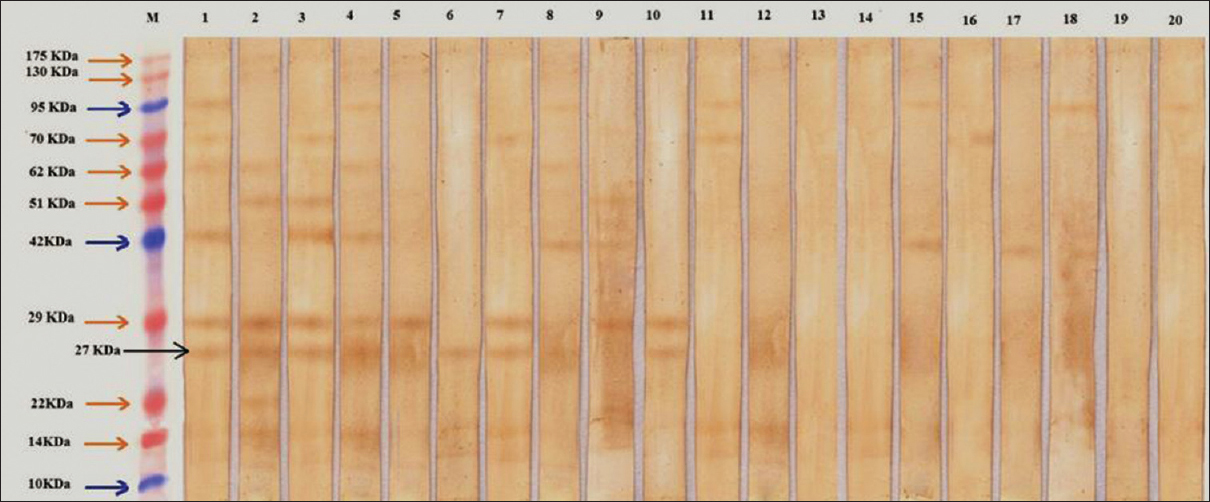
- Immunoblotting of somatic antigens of Fasciola hepatica with sera from fascioliasis and nonfascioliasis patients and healthy controls. M: Protein marker, lane 1–10 sera from fascioliasis patients; lane 11–20 sera from nonfascioliasis and healthy controls
Although a 29 kDa antigen was also detected in the sera of most of the fascioliasis patients, however its specificity was not as high as the 27 kDa antigen.
The 27 kDa antigen was purified from the SA and was used in an indirect ELISA system for the diagnosis of human fascioliasis. Of 15 sera of fascioliasis patients, all (100%) were found to be positive by ELISA while only 4 cases (6.25%) of nonfascioliasis patients (32 cases) were false-positive by this system. Sera from healthy controls (30 cases) did not react with the antigen and were all negative. Accordingly the sensitivity and specificity of the test were 100% (95%, CI: 84–100%) and 93.6% (95% CI: 83–97%) respectively. Table 1 shows the performance of ELISA, using the 27 kDa antigen, in diagnosis of human fascioliasis.

DISCUSSION
Human fascioliasis, as a public health problem, has been reported from different part of the world.[1] It is necessary to develop and establish a reliable, simple and rapid diagnostic tool for proper diagnosis of this zoonotic infection especially in endemic areas. Common laboratory diagnosis of fascioliasis are mainly relied on detection of anti-Fasciola antibodies. A variety of methods have been developed and used for diagnosis of human fascioliasis, among them ELISA and immunoblotting are the most considered ones.[1415161718]
In the present study, we evaluated the performance of a 27 kDa subunit of F. hepatica SAs in two systems, immunoblotting and ELISA, and showed that this subunit has satisfactory validity in diagnosis of human fascioliasis.
Serological diagnosis of human fascioliasis, based on fractions of adult worm antigens, has been reported in different studies.[812141718] Since F. hepatica is the main cause of human fascioliasis, most of the studies related to diagnosis of fascioliasis are focusing on subunits purified from either somatic or ES antigens of this species of the fluke. Special attention has been given to the 23–40 kDa subunits of F. hepatica.[14161920] Shaker et al., reported that antigenic fractions of 33 and 54 kDa of Fasciola SAs have the sensitivity and specificity of 100 and 93%.[21] Maleewong et al., reported a sensitivity of 100% and specificity of 96% for a 38 kDa subunit of F. gigantica adult worms.[22] In the agreement with the previous studies, we found that a 27 kDa of F. hepatica, purified from the adult SAs, has suitable performance in diagnosis of fascioliasis. Our finding is also in agreement with a previous study, which showed that a 27 kDa antigen of F. gigantica adult worm has a valuable performance in an ELISA system for the serodiagnosis of human fascioliasis.[14]
CONCLUSION
Findings of this study demonstrated that both Western blotting and the indirect ELISA, based on the 27 kDa subunit of F. hepatica SA, are reliable methods for serodiagnosis of human fascioliasis. As the Western blotting is much more labor-intensive than the ELISA, the use of ELISA as a screening method and Western blotting as confirmation assay can be suggested. Studies with larger number of positive samples and also samples from patients with different helminthic infections are needed to validate the performance of this subunit in diagnosis of human fascioliasis. Biochemical nature and function of this protein are yet to be known.
ACKNOWLEDGEMENTS
The results described in this paper were part of PhD thesis of Reza Shafiei. The study was financially supported by the office of vice-chancellor for research of Shiraz University of Medical Sciences (Grant No. 90-5750). Technical assistance of Miss. Zahra Yosefi is greatly acknowledged.
Source of Support: Office of vice-chancellor for research of Shiraz University of Medical Sciences (Grant No. 90-5750).
Conflict of Interest: None declared.
REFERENCES
- Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv Parasitol. 2009;69(Chapter 2):41-146.
- [Google Scholar]
- Fascioliasis: US, CT, and MRI findings with new observations. Abdom Imaging. 2000;25:400-4.
- [Google Scholar]
- Immunodiagnosis of human and animal fascioliasis. In: Dalton JP, ed. Fasciolosis. Oxon, Wallingford, London, UK: CABI Publishing; 1998. p. :435-48.
- [Google Scholar]
- Immunodiagnosis of Fasciola hepatica infection (fascioliasis) in a human population in the Bolivian Altiplano using purified cathepsin L cysteine proteinase. Am J Trop Med Hyg. 1998;58:417-23.
- [Google Scholar]
- Evaluation of Fas2-ELISA for the serological detection of Fasciola hepatica infection in humans. Am J Trop Med Hyg. 2007;76:977-82.
- [Google Scholar]
- Short report: Immunodiagnosis of human fascioliasis using recombinant Fasciola hepatica cathepsin L1 cysteine proteinase. Am J Trop Med Hyg. 1999;60:749-51.
- [Google Scholar]
- Comparison of adult somatic and cysteine proteinase antigens of Fasciola gigantica in enzyme linked immunosorbent assay for serodiagnosis of human fasciolosis. Acta Trop. 2003;88:69-75.
- [Google Scholar]
- Diagnosis of human fasciolosis in the Gilan province of Northern Iran: Application of cathepsin L-ELISA. Diagn Microbiol Infect Dis. 2002;44:175-9.
- [Google Scholar]
- Evaluation of fast-ELISA versus standard-ELISA to diagnose human fasciolosis. Arch Iran Med. 2011;14:18-21.
- [Google Scholar]
- A consistent PCR-RFLP assay based on ITS-2 ribosomal DNA for differentiation of Fasciola species. Iran J Basic Med Sci. 2013;16:1266-9.
- [Google Scholar]
- Application of western blotting using native antigen B for serodiagnosis of human cystic echinococcosis. Iran J Parasitol. 2007;2:7-12.
- [Google Scholar]
- Seroprevalence of human fasciolosis in a new-emerging focus of fasciolosis in Yasuj district, southwest of Iran. Iran J Parasitol. 2012;7:15-20.
- [Google Scholar]
- Immunodiagnosis of human fascioliasis using an antigen of Fasciola gigantica adult worm with the molecular mass of 27 kDa by a dot-ELISA. Southeast Asian J Trop Med Public Health. 2003;34:713-7.
- [Google Scholar]
- Fasciola hepatica cysteine proteinases: Immunodominant antigens in human fascioliasis. Am J Trop Med Hyg. 1997;57:660-6.
- [Google Scholar]
- Usefulness of 8 kDa protein of Fasciola hepatica in diagnosis of fascioliasis. Korean J Parasitol. 2003;41:121-3.
- [Google Scholar]
- Fasciola hepatica saposin-like protein-2-based ELISA for the serodiagnosis of chronic human fascioliasis. Diagn Microbiol Infect Dis. 2011;70:355-61.
- [Google Scholar]
- Diagnostic efficacy of monoclonal antibody based sandwich enzyme linked immunosorbent assay (ELISA) for detection of Fasciola gigantica excretory/secretory antigens in both serum and stool. Parasit Vectors. 2011;4:176.
- [Google Scholar]
- Partially purified fraction (PPF) antigen from adult Fasciola gigantica for the serodiagnosis of human fascioliasis using Dot-ELISA technique. Ann Saudi Med. 2004;24:18-20.
- [Google Scholar]
- A cysteine proteinase from the liver fluke Fasciola spp.: Purification, characterization, localization and application to immunodiagnosis. Jpn J Parasitol. 1989;38:374-84.
- [Google Scholar]
- Evaluation of specific Fasciola antigen in the immunodiagnosis of human fascioliasis in Egypt. J Egypt Soc Parasitol. 1994;24:463-70.
- [Google Scholar]
- Antigenic components of somatic extract from adult Fasciola gigantica recognized by infected human sera. Asian Pac J Allergy Immunol. 1997;15:213-8.
- [Google Scholar]





