Translate this page into:
Performance of modified colistin broth disc elution vis-a-vis broth microdilution method for susceptibility testing of Enterobacterales
*Corresponding author: Ashoka Mahapatra, Department of Microbiology, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India. micro_ashoka@aiimsbhubaneswar.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Kar P, Mahapatra A, Malik BA, Behera B, Mohanty S. Performance of modified colistin broth disc elution vis-a-vis broth microdilution method for susceptibility testing of Enterobacterales. J Lab Physicians. 2024;16:286-90. doi: 10.25259/JLP-2023-5-25-(1801)
Abstract
Objectives:
Recently, Clinical and Laboratory Standards Institute (CLSI) has approved colistin broth disc elution (CBDE) to be a supplemental test. This requires multiple discs and tubes to get the desired concentrations of colistin -1, 2, and 4 µg/mL and 10 mL volume of cation-adjusted Mueller–Hinton broth for a single isolate. The present study was aimed to evaluate the performance of CBDE in a microtiter plate format modified (mCBDE) with the reference method broth microdilution (BMD) for detection of colistin resistance in carbapenem-resistant Enterobacterales (CRE) isolates.
Materials and Methods:
One hundred and sixty non-duplicate clinical CRE isolates (May 2021–April 2022) were simultaneously subjected for BMD and mCBDE. For mCBDE, colistin 10 µg discs and Mueller–Hinton broth no-2 control cations were procured from HiMedia, Mumbai, and drug concentrations were prepared following CLSI-M100Ed31. Results of mCBDE were compared with reference BMD (Minimum inhibitory concentration [MIC] ≤2 µg/mL – intermediate and ≥4 µg/mL – resistant).
Statistical Analysis:
The performance of mCBDE was compared with BMD and expressed in terms of Categorical, essential agreement (EA), very major error (VME), and major error (ME). The sensitivity and specificity were calculated using Fisher’s contingency Table.
Results:
Of the 160 CRE isolates, 152 had exactly the same minimal inhibitory concentration (MIC) in both the tests with four isolates having higher and four having lower colistin MIC by mCBDE, giving a major error of 2.1% and VME of 5.5%. Categorical and essential agreement of mCBDE were 97.5% and 98.7%, respectively.
Conclusions:
mCBDE is an easy, economical, and reliable alternative test for determining colistin susceptibility for CRE isolates. Further, large-scale study is needed to strengthen our observation.
Keywords
Carbapenem-resistant Enterobacterales
Colistin
Colistin broth disc elution
modified colistin broth disc elution
Broth microdilution
INTRODUCTION
Carbapenem-resistant Gram-negative bacteria have become a global threat, and polymyxins are the most relied option for them.[1] In the year 2017, WHO declared colistin as one of the drugs in the “RESERVE” category, which can only be used in the most severe circumstances when all other alternatives have failed.[2] However, resistance to polymyxin is also increasing. Only broth microdilution (BMD) is recommended by the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) for polymyxin susceptibility testing, which is impractical for most clinical microbiology laboratories.[3,4] Most clinical microbiology laboratories rely on disk diffusion or gradient diffusion susceptibility testing methods, but unfortunately, the polymyxins, being large cationic molecules, diffuse poorly, and there are high rates of very major errors (VMEs) as an isolate is that is read susceptible when it is resistant by the recommended BMD method. Most clinical microbiology laboratories are unable to provide an accurate colistin susceptibility report to clinicians due to the lack of a simple and easy-to-perform colistin susceptibility test method. Nordmann et al. have developed a simple and rapid polymyxin NP test as an alternative to BMD for screening of colistin-resistant Enterobacterales (CoRE) isolates.[5] Although rapid, this test requires fresh preparation of colistin-containing solution that has a maximum shelf-life of 72 h and appropriate storage. This also fails to detect the minimum inhibitory concentration (MIC) of colistin, and only detect colistin resistance qualitatively. Simner et al. had reported an easy and practical method to perform colistin MIC testing by colistin broth disk elution (CBDE) test for colistin susceptibility testing of Gram-negative bacteria and got an encouraging result of 98% categorical agreement (CA), 99% essential agreement (EA), and no major errors (MEs) when compared to reference BMD method.[6] However, the limitations of this method are use of 0, 1, 2, and 4 numbers of colistin (10 µg) disks in four different 10-mL cation-adjusted Mueller–Hinton broth (CAMHB) tubes, to get a presumed colistin concentrations in the tubes to be 0, 1, 2, and 4 µg/mL/mL, respectively. Thus, for each isolate, a minimum of 7 colistin discs and five tubes of 10 mL capacity containing CAMHB medium are exhausted. To achieve the same drug concentration in the lower volumes, the present study was planned to use a modified CBDE (mCBDE) test in a single set of CBDE test materials by performing in a 96-well microtiter plate so that multiple isolates can be tested at once for colistin susceptibility using a single set. Thus, there shall be less labor and use of a smaller number of tubes, colistin discs, and CAMHB medium and will be compared simultaneously with reference BMD. The results will be helpful for the adoption of a simpler and cost-effective method for colistin susceptibility testing in routine practice.
MATERIALS AND METHODS
The proposed work was a retrospective evaluation of CBDE test in a microtiter plate for Colistin Susceptibility Testing of clinical isolates of multidrug resistant (MDR) Enterobacterales.
Study design
This was a retrospective observational study.
Study subjects
Isolates of MDR Enterobacterales from routinely received clinical specimens in the Department of Microbiology in a tertiary care institute during a period of one year (May 1, 2021–April 30, 2022).
All the isolates were subjected simultaneously to reference BMD as per standard procedure and modified colistin broth disc elution as described below.
Method for modified colistin broth disc elution test
A single set of tests containing 1, 2, and 4 numbers of colistin (10 µg, HIMEDIA, MUMBAI) disks in three different tubes with 10-mL single strength (1X) CAMHB (HIMEDIA, MUMBAI) were prepared along with one growth control without any colistin disc to get a presumed colistin concentrations of 0, 1, 2, and 4 µg/mL in the tubes, respectively. Then, tubes were incubated at room temperature for 30 min to allow the colistin to elute from the disks and attain the presumed colistin concentrations to be of 0, 1, 2, and 4 µg/mL in the respective tubes. The microtiter plate was labeled as per drug concentration, and to each row 200 µLt of the desired concentrations (0, 1, 2, and 4 µg/mL) of CAMHB with colistin eluted were added. Standard inoculum (0.5 McFarland) of the test organisms in 3 µL quantity was added to each well and incubated overnight. Positive control [National Collection of Type Cultures (NCTC) Escherichia coli 13846] and negative controls [American Type Culture Collection (ATCC) E. coli 25922] were put up with each set. The plate was incubated overnight at 37°C. The MIC was taken as the tube having the lowest concentration and no visible turbidity and interpreted to be resistant if >4 µg/mL and intermediate if <2 µg/mL.[7] The MIC results of mCBDE were compared with the reference BMD method. The agreement between the BMD and mCBDE method was expressed in terms of categorical agreement (CA) essential agreement (EA). CA was calculated as the percentage of isolates with results in the same category as the reference method, taking all isolates tested as denominator (n = 160). EA was calculated as the percentage of isolates that had MIC values within ±1 log2 dilution or ±1 two-fold dilution of the reference standard. As per CLSI, any test with CA and EA >90% is reliable and can be used as an alternative to the reference test.[8]
ME and VME were calculated for mCBDE test. Isolates showing MIC in the intermediate range by the reference BMD and resistant range in the new method under evaluation were taken as minor error/ME (false resistance), whereas isolates showing MIC in the resistant range by reference BMD and sensitive range in the new method were taken as VME (false susceptible). Acceptable ME and VME rates are <3% of the susceptible and resistant isolates tested.[9]
RESULTS
In the present study, we have included a total of 160 non-duplicate Carbapenem resistant Enterobacterales (CRE) isolates obtained from various clinical samples received for routine culture and susceptibility test. As the study was conducted during the period of the ongoing COVID pandemic, of the 160 CRE isolates, the majority were recovered from inpatients (159/160, 99.3%), and 46.2% of them (74/160) were obtained from various intensive care units. Among the 160 CRE isolates, the majority were obtained from respiratory samples (70/160, 43.7%), followed by urine (37/160, 23.1%), blood (26/160, 16.2%), pus (21/160, 13.1%), and sterile body fluids (6/160, 3.7%). All the isolates were simultaneously subjected to BMD and mCBDE tests for colistin susceptibility. The results for positive and negative controls were satisfactory. Among these 160 isolates, 88.75% (142/160) were intermediate susceptible (MICs ≤2 µg/mL), and 11.2% (18/160) were resistant (MIC from >4 µg/mL) to colistin according to the reference BMD test. The susceptible isolates included 59.2% (84/142) Klebsiella spp., 39.4% (56/142) E. coli, and 1.4% (2/142) Enterobacter spp. and the resistant isolates were 55.5% (10/18) Klebsiella spp., 33.3% (6/18) E. coli, and 11.1% (2/18) were Enterobacter spp. Similarly, 87.5% (140/160) isolates were found to be intermediate susceptible including 57.8% (81/140) Klebsiella spp., 40.7% (57/140) E. coli, and 1.4% (02/140) Enterobacter spp. and 12.5% (20/160) were resistant to colistin including 65% (13/20) Klebsiella spp., 25% (5/20) E. coli, and 10% (2/20) Enterobacter spp. by mCBDE test. Seventeen isolates were resistant, and 139 were intermediate susceptible by both methods, showing the same profile.
Individual performance characteristics of mCBDE compared to BMD
The agreement analysis of mCBDE to BMD is depicted in Figure 1a. The number of isolates with the same MIC was shown in yellow box, whereas ± 1 log2 and ± 2 log2 MIC values were shown in light brown box and light blue box respectively. These values are used for extrapolating the CA and EA. In our study, mCBDE showed a CA and EA of 97.5% and 98.7%, respectively. Of the 160 isolates tested, 152 had exactly the same MIC as that of BMD. Among the rest, eight isolates, six had MIC within ±1 log2 dilutions and two had higher MIC (8 µg/mL) compared to BMD (1 µg/mL). Out of 142 colistin intermediate susceptible isolates, three showed ME (03/142, 2.1%) or false resistance. Similarly, out of 18 colistin-resistant isolates, one showed VME or false susceptibility in mCBDE (01/18, 5.5%). The sensitivity and specificity of mCBDE test as compared to reference BMD were found to be 97.8% and 94.4%, respectively [Figure 1b].
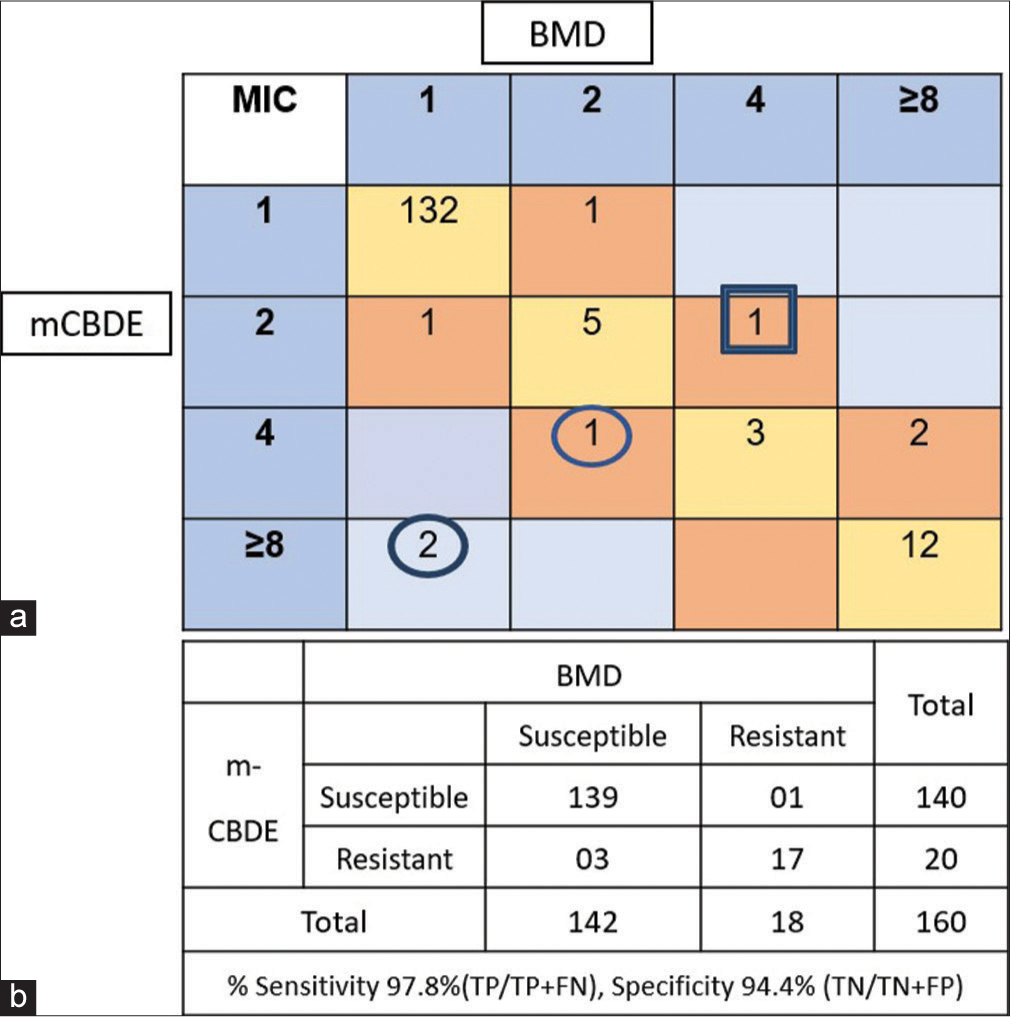
- Performance of modified colistin broth disc elution (mCBDE) compared to broth microdilution (BMD)- (n = 160). (a) Categorical agreement analysis of minimum inhibitory concentration values of colistin in broth microdilution (BMD) versus mCBDE and (b) performance of mCBDE compared to BMD (n = 160). Circle denotes isolates showing major error (ME), where as Square denotes very major error (VME). TP: True positive, FN: False negative.
DISCUSSION
Colistin, a five-decade-long antimicrobial, regained a cult status in the 21st century to address the growing menace of infection due to CRE. Although the pharmacokinetics and pharmacodynamics of polymyxins are still in debatable status, there is an increase in usage of colistin due to the lack of availability of alternative antimicrobials, leading to emergence of colistin-resistant pathogens.[9] A rapid colistin susceptibility test is highly needed in diagnostic clinical microbiology laboratories, because the joint CLSIEUCAST recommended method (BMD) is time-consuming (>48 h), expensive, and demands expertise, thus difficult to implement in routine practices.[10] Over the years, many studies have evaluated colistin susceptibility by automated system, agar dilution, E-test, and disk diffusion, considering BMD as the gold standard. However, most of them have reported high error rates in all these methods. Khurana et al. had reported the CA to be 88% (798/910) with 10% (95/910) VME and 1% (9/910) ME for Gram-negative bacilli by the VITEK 2 system.[11] Kar et al. also reported high VME rates for both agar dilution (11%) and E-test (37%) in Enterobacterales.[12] A rapid colorimetric assay for polymyxin susceptibility (NP test) by Nordmann et al. gained popularity as one of the screening tests with a sensitivity and specificity of >95%.[5] However, the disadvantage of NP test was, its performance is limited to the Enterobacterales only.[13] Kar et al. could accurately detect 26/31 CoRE by polymyxin NP test with an overall CA of 83.8%, but failed to detect five Enterobacter spp. 5/31 (16.1%) with overall VME to be 16%,which decreased to <3% when those Enterobacter spp. (n = 5) were excluded from the study.[14]
Recently, the CLSI antimicrobial susceptibility testing subcommittee endorsed the CBDE and colistin agar test (CAT-10) methods for colistin susceptibility testing of Enterobacterales and Pseudomonas aeruginosa based on the results of CBDE (97.9% CA and 94.4% EA) and CAT-1/CAT-10 (99.4% CA and 99.7% EA) as compared to reference BMD.[9] Unlike polymyxin NP test, CBDE can also be used for glucose-non-fermenting organisms. Although both CBDE and CAT are showing excellent agreement with BMD, CBDE is gaining popularity compared to CAT as the former is simpler and easy to perform.[9]
In our study, among the 160 isolates, 88.75% (142/160) were intermediate susceptible (MICs ≤2 µg/mL), and 11.2% (18/160) were resistant (MIC >4µg/mL) to colistin according to the reference BMD test.
Among the 17 CoRE detected by both BMD and mCBDE, Klebsiella pneumonia was the major isolates (58.5%, 10/17) with higher MIC value (≥8 µg/mL). In our study, the result of mCBDE was satisfactory with a CA and EA of 97.5% and 98.7%, respectively. The ME of mCBDE was within the acceptable range (2.1%), whereas VME was high (5.5%). Dalmolin et al.[15] had also performed CBDE in diminished volumes of the reagents (colistin broth microdilution) with a final volume of 1 mL and the microelution-plates test – final volume of 200 µL on 85 g-negative rods obtained from two different research centers in Brazil. They had evaluated one more modified method – colistin susceptibility test tube to overcome the issue of adhesion to the microelution-plates. They performed the test in the same manner in one glass tube (5 mL of CA-MHB + 10 µg colistin disk which gave concentration of 2 µg/mL and to that 25 µL of test inoculum [108 CFU/mL] added) and interpreted resistant when growth (turbidity) was observed (MIC >2 µg/mL). They reported satisfactory results for Enterobacterales with fair agreement, sensitivity, and specificity by all three methods, but the major and VME were less satisfactory. However, their results for non-fermentative isolates were not satisfactory, the agreement, sensitivity, and specificity were low and error rates were high.[15] The possible reason for high error rates was hypothesized by various authors that the assumed colistin concentrations eluted from the discs in the broth medium may not be as accurate as desired, thus leading to the high VME.[15] Further studies are needed to measure the exact concentration of polymyxin in the broth after elution.
Ngudsuntia et al.[16] evaluated the performance of CBDE and rapid colistin disk elution (RCDE) test for Gram-negative bacilli as compared to the reference BMD test. They performed RCDE test using a 10-µg colistin disk in 2.7 mL volume (final colistin concentration-3.7 µg/mL) of CAMHB or phenol red broth base, added 1-µL loop of bacterial inoculums, incubated 1–4 h. for Enterobacterales and 16–20 h. for Acinetobacter baumannii. For Enterobacterales, the CA and VME rate were 98.3% and 5.4%, respectively, in the RCDE test and 97.9% and 7.1%, respectively, in the CBDE test with a ME of 0.6% in both the tests. However, for the A. baumannii isolates, the RCDE and CBDE tests showed high VME rates (8.3% and 16.7%, respectively). As per their observation, the performance of RCDE was good and comparable with CBDE; moreover, it was cheaper, more rapid (3 h), and convenient, thus suggesting RCDE to be an alternative for detecting colistin resistance among Enterobacterales in low-income countries.[16]
The ad hoc working group reported low performance of QC strain (E. coli ATCC 25,922) used in CBDE, thus CLSI recommended E. coli AR Bank no. 0349 (mcr1 positive) as the QC strain.[9] We had used NCTC E. coli 13846 (mcr1) as a positive control and got satisfactory results.
The mCBDE conducted at our setup had solved the issues raised by Simner et al. regarding the use of numerous discs, tubes, and occupying more space in incubator for CBDE.[6]
CONCLUSIONS
This is the first report from Eastern India to perform modified CBDE in reduced volume. Although there is no specific recommended brand of colistin disc/CAMHB, we used only one brand (HiMedia) and got excellent sensitivity (97.8%) and specificity (94.4%). Thus, mCBDE can be used as an alternative to CBDE to save a lot of resources.
The limitations of our study were small sample size, and non-inclusion of medically important non-fermenters (Pseudomonas spp., Acinetobacter spp.). Moreover, the mCBDE could have been compared with that of CBDE for agreement analysis. Further, it is suggested to perform the tests by two different persons to ascertain its repeatability and reproducibility.
Ethical approval
As the study was conducted on retrospectively isolated bacteria from routine clinical specimens, approval was obtained for exemption from the review process. Hence Institutional Review Board approval was not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
Dr. Bijayini Behera is on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The search for a practical method for colistin susceptibility testing: Have we found it by going back to the future? J Clin Microbiol. 2019;57:e01608-18.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: https://www.who.int/news/item/06-06-2017-who-updates-essential-medicines-list-with-new-advice-onuse-of-antibiotics-and-adds-medicines-for-hepatitis-c-hiv-tuberculosis-and-cancer [Last accessed on 2023 Dec 13]
- Performance standards for antimicrobial susceptibility testing; 28th informational supplement In: CLSI M100-S28. Wayne, PA: Clinical and Laboratory Standards Institute; 2018.
- [Google Scholar]
- Breakpoint tables for interpretation of MICs and zone diameters, version 8.1. 2018. Available from: https://www.eucast.org/clinical_breakpoints [Last accessed on 2023 Dec 13]
- [Google Scholar]
- Rapid detection of polymyxin resistance in Enterobacterales. Emerg Infect Dis. 2016;22:1038-43.
- [CrossRef] [PubMed] [Google Scholar]
- Two-site evaluation of the colistin broth disk elution test to determine colistin in vitro activity against gram-negative Bacilli. J Clin Microbiol. 2019;57:e01163-18.
- [CrossRef] [PubMed] [Google Scholar]
- Performance standards for antimicrobial susceptibility testing In: CLSI supplement M100 (30th ed). Wayne, PA: Clinical and Laboratory Standards Institute; 2020.
- [Google Scholar]
- Comparative evaluation of broth microdilution with polystyrene and glass-coated plates, agar dilution, E-Test, vitek, and disk diffusion for susceptibility testing of colistin and polymyxin B on carbapenem-resistant clinical isolates of Acinetobacter baumannii. Microb Drug Resist. 2018;24:1082-8.
- [CrossRef] [PubMed] [Google Scholar]
- Multicenter evaluation of colistin broth disk elution and colistin agar test: A report from the clinical and laboratory standards institute. J Clin Microbiol. 2019;57:e01269-19.
- [CrossRef] [PubMed] [Google Scholar]
- Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev. 2017;30:557-96.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of Vitek®2 performance for colistin susceptibility testing for Gram-negative isolates. JAC Antimicrob Resist. 2020;2:dlaa101.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of colistin resistance in carbapenem resistant Enterobacteriaceae by reference broth microdilution and comparative evaluation of three other methods. J Lab Physicians. 2021;13:263-9.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the rapid polymyxin NP Test for polymyxin B resistance detection using Enterobacter cloacae and Enterobacter aerogenes isolates. J Clin Microbiol. 2017;55:3016-20.
- [CrossRef] [PubMed] [Google Scholar]
- Performance evaluation of colistin rapid NP test for detection of colistin resistance in colistin resistant Enterobacterales. Med J Armed Forces India 2022 doi: 10.1016/j.mjafi.2022.04.006
- [CrossRef] [PubMed] [Google Scholar]
- Elution methods to evaluate colistin susceptibility of Gram-negative rods. Diagn Microbiol Infect Dis. 2020;96:114910.
- [CrossRef] [PubMed] [Google Scholar]
- Colistin susceptibility testing by rapid colistin disk elution test among Enterobacteriaceae in low-resource setting. Microb Drug Resist. 2021;27:1685-91.
- [CrossRef] [PubMed] [Google Scholar]






