Translate this page into:
Prognostic significance of Bcl-2 expression in carcinoma of the uterine cervix: A systematic review and meta-analysis
*Corresponding author: Saikat Das, Department of Radiation Oncology, All India Institute of Medical Sciences, Bhopal - 462020, Madhya Pradesh, India. saikat.radiotherapy@aiimsbhopal.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Das S, Jeyaseelan V, Nadaraj A, Mukhopadhyay S, Agrawal A, John S. Prognostic significance of Bcl-2 expression in carcinoma of the uterine cervix: A systematic review and meta-analysis. J Lab Physicians. doi: 10.25259/JLP_45_2024
Abstract
Apoptosis is the final common pathway in cellular death induced by radiation and chemotherapy. Antiapoptotic protein bcl-2 plays an essential role in the determination of cellular threshold in the process of apoptosis. Immunohistochemical evaluation of bcl-2 has been one of the most widely investigated prognostic markers in cervical cancer. Given the lack of conclusive evidence in the literature, we aimed to systematically review the evidence to use bcl-2 as a prognostic marker for overall (OS) and disease-free survival (DFS) in cervical cancer. We reviewed the studies after a systematic literature search, reporting either OS or DFS. Estimates were extracted from these studies, and a meta-analysis was done. Positive bcl-2 expression was associated with a decreased risk ratio (RR) for OS. The estimated log RR was −0.52 (confidence interval = −0.91–−0.13, RR = 0.60\0.40, 0.88], P < 0.001). No significant association was found with DFS. There was significant heterogeneity among the studies. Bcl-2 can be used as a molecular marker for OS in cervical cancer. It can be helpful to identify a group of patients who might be good responders in locally advanced cervical cancer and help in clinical decision-making to prognosticate the disease.
Keywords
Uterine neoplasms
bcl-2
Apoptosis
Cervical cancer
Chemoradiotherapy
INTRODUCTION
Cervical cancer is one of the most common malignancies in low and middle-income countries.[1] Given the high disease burden and scarcity of available treatment facilities, prognostication of disease is essential. Recently, Noordhuis et al. reviewed the prognostic significance of various cell biological markers for patients treated with radiation with or without chemotherapy.[2] Radiation or chemotherapy leads to the induction of multiple molecular pathways, which ultimately culminate in cell death through apoptosis.[3] The apoptotic threshold is related to the balance between proapoptotic (BAX or Bcl-2 associated X protein, BAK or Bcl-2 antagonist killer) and anti-apoptotic group of proteins (FLICE inhibitory proteins, Bcl-2, and IAP or inhibitors of apoptosis protein).[4] Literature evidence suggests that biomarkers for apoptosis are related to prognosis in breast cancer, lung cancer, and lymphoma.[5,6] Among all these markers, the Bcl-2 homology domain (BH) containing proapoptotic (BAX) and anti-apoptotic proteins (Bcl-2) are the primary determinants of the cellular apoptotic process.[4] The biological activity of these proteins is determined by their ability to form homo or heterodimers and regulate the mitochondrial membrane permeability and electrical conductance.
Bcl-2 is an antiapoptotic protein or death antagonist in the mitochondrial membrane, endoplasmic reticulum, and nuclear envelope.[7] In normal cervical epithelium, it is shown to be localized in the basal layer, and increased expression has been reported in cervical intraepithelial neoplasia (CIN).[8,9] Expression of bcl-2 has been linked to the progression of premalignant cervical lesions to invasive malignancy.[10] Various studies have evaluated the prognostic value of bcl-2 as a predictor of treatment outcomes in cervical cancer, and the immunohistochemical method has been used in most of the studies.[11-13] The prognostic role of bcl-2 expression on treatment outcome is still controversial.[14] There is significant heterogeneity concerning the biomarker evaluation, threshold value, and quantitative or qualitative assessment method. Here, we report a systematic review and meta-analysis of the role of bcl-2 in the prognosis of cervical cancer based on available literature.
MATERIALS AND METHODS
Search strategy
This systematic review and meta-analysis were aimed to address the research question “What is the prognostic significance of immunohistochemical expression of bcl-2 in cervical cancer?” and were conducted as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[15] The search strategy and keywords used are tabulated in Table 1. The following medical literature databases were searched: PubMed, Scopus, Cochrane Library, and ScienceDirect (the last search was done on 12th January 2024). We manually checked the reference list of all the identified articles and were manually searched for any additional studies.
| Search set | PubMed |
|---|---|
| 1 | Bcl2 |
| 2 | BCL2 |
| 3 | Bcl |
| 4 | bcl* |
| 5 | Bcell CLL/lymphoma 2 |
| 6 | APOPTOSIS REGULATORY PROTEINS |
| 7 | APOPTOSIS |
| 8 | UTERINE CERVICAL NEOPLASM |
| 9 | 1–7/OR |
| 10 | 8 AND 9 |
| 11 | prognos* |
| 12 | 10 AND 11 |
CLL: Chronic lymphoid leukemia. Words in capital letters are MeSH terms.
SCOPUS SEARCH: (TITLEABSKEY (“bcl”) AND TITLEABSKEY (“cervical cancer”)) AND ( (prognos*) ) AND (LIMITTO (EXACTKEYWORD, “Human”) OR LIMITTO (EXACTKEYWORD, “Female”) OR LIMITTO (EXACTKEYWORD, “Humans”) OR LIMITTO (EXACTKEYWORD, “Protein bcl 2”) OR LIMITTO (EXACTKEYWORD, “Uterine Cervical Neoplasms”) OR LIMITTO (EXACTKEYWORD, “Uterine cervix cancer”)), *wildcard symbol that broadens a search by finding words that start with the same letters.
Inclusion and exclusion criteria
For inclusion in the meta-analysis, the following inclusion criteria were laid down: (1) Studies that described the treatment outcome of patients with invasive cervical cancer (treatment could be surgery, radiotherapy, and or chemotherapy), (2) studies were data on pre-treatment expression level of Bcl-2 from the primary tumor was available, (3) retrospective or prospective study (cohort, randomized studies were included), (4) data should be sufficient to allow estimation of risk ratio (RR), and (5) studies published in English language only were included. Letters to editors, review articles, papers published in languages other than English, and papers published in books were excluded from the meta-analysis.
Data extraction
The data collection form was designed to collect relevant information from the selected studies. If an article was not selected, then the reason for exclusion was documented. The following data were extracted from each study: the surname of the first author, year of publication, period of recruitment, the country where the study was conducted, the total number of patients, stage and grade of the tumor, and the treatment given. Data regarding the method of assessment of prognostic factor (bcl-2) were collected, as well as criteria for determining the positivity of the biomarker wherever available. The primary outcomes were entered in the form of a table showing the overall (OS) and disease-free survival (DFS) with respect to bcl-2 expression.
Statistical analysis
The outcome measure considered for the data pooling was the log risk ratio (lnRR) and its variance. The lnRR was calculated for each study based on the deaths in each group and the total number of subjects studied in each group. When the number of deaths was not given directly, it was estimated using the survival probability provided in each group. The random effects model approach was used to calculate a weighted estimate of the treatment effect across the studies. The heterogeneity among studies was assessed through a χ2 statistic with n-1 degrees of freedom (I2), and if the results of studies were heterogeneous (I2 > 70%), the effects of possible explaining factors were explored. All calculations and statistical tests were done using R (Version 3.2.0) software.
RESULTS
Eligible studies
A total of 292 articles were retrieved by electronic search. After removing duplicates using the search criteria as shown in Table 1. A total of 24 studies were assessed for eligibility after initial screening and reviewing the abstract. Among these studies, 16 were included in the meta-analysis[10,13,16-29] [Figure 1]. The sixteen studies’ characteristics are summarized in Table 2. These studies reported at least one of the survival outcomes of interest (14 studies reported overall, and eight studies reported DFS) and were selected for meta-analysis. The sample size of these included studies ranged from 29 to 259 (mean 101). Total number of patients included in all the studies was 1612. Immunohistochemistry (IHC) to detect bcl-2 was done upfront before starting treatment in all these studies. The staining pattern, percentage of patients who were positive for bcl-2, and the cutoff value, as reported in the studies, are presented in Table 3. The average prevalence of bcl-2 positivity in the entire population was 49.2% (10–75%).
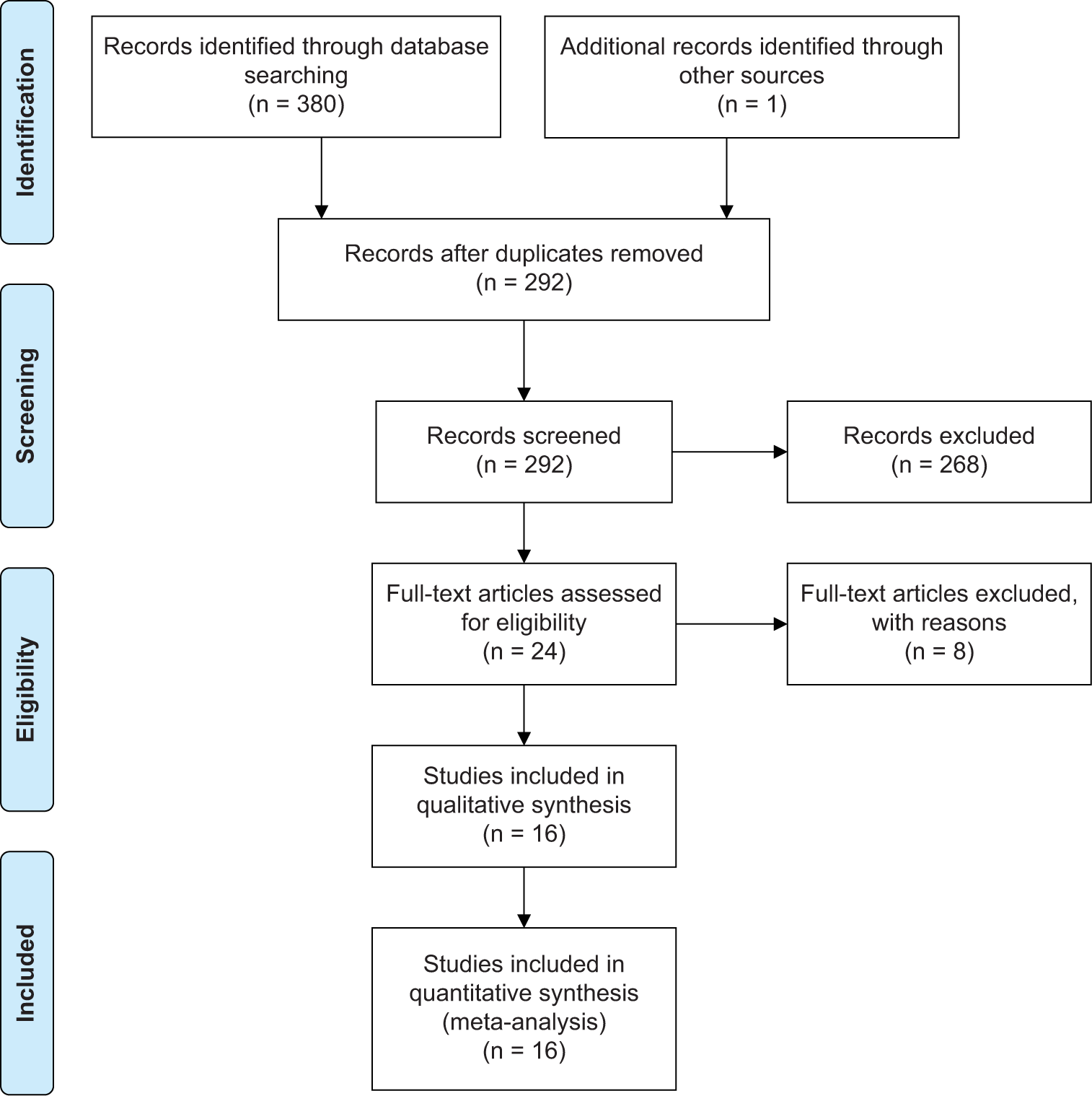
- Preferred reporting items for systematic reviews and meta-analyses chart of included studies. #: number of.
| Authors | Country | Number | % of bcl-2 positivity | Cut off value of IHC | Stage | Method of IHC (1=semiquantitative, 2 = quantitative) |
Treatment | Outcomemeasure |
|---|---|---|---|---|---|---|---|---|
| Uehara, 1995 | Japan | 259 | 33 | NA | Ib, IIa, IIb | 1 | Surgery (Radiation for LN + cases) | OS |
| Tjalma, 1997 | Belgium | 76 | 63 | 5 | IaIIb | 2 | Wertheim’s radical Hysterectomy (RT for LN/LVSI+) | OS |
| Crawford, 1998 | England | 44 | 10 | I–IV | 2 | - | OS | |
| Harima, 1998 | Japan | 44 | 61 | 30 | Ib–IVb | 2 | RT | OS |
| Rajkumar, 1998 | India | 40 | 65 | IIB and IIIB | 1 | RT | OS, DFS | |
| Padovan, 2000 | Italy | 86 | 60 | 5 | Ib1–IVb | 2 | Surgery | OS |
| Tjalma, 2001 | Belgium | 111 | 68 | 5 | Ia–IVb | 2 | NM | OS |
| Chung, 2002 | Hong Kong, China | 43 | 65 | 10 | I–IV | 2 | - | DFS |
| Grauflund, 2002 | Sweden | 168 | 44 | 30 | I–II | 2 | Surgery (adjuvant RT if LVI, para+, LN+) | OS, DFS |
| Jain, 2003 | India | 76 | 38 | NA | IIb–III | 1 | RT | OS, DFS |
| Wootipoom, 2004 | Thailand | 174 | 10 | 50 | Ib–IV | 2 | RT | OS, DFS |
| Saito, 2004 | Japan | 29 | 48 | 10 | Ib–IVb | 2 | Surgery/RT | OS |
| Munakata, 2005 | Japan | 125 | 75 | 10 | I–IV | 2 | Surgery ± RT ± Chemo | OS, DFS |
| Van de Putte, 2005 | Norway | 220 | 27 | 5 | Ib | 2 | RT | OS, DFS |
| Yamasita, 2009 | Japan | 57 | 19 | NA | II, III, IVA | 2 | RT | OS |
| Henriquez, 2011 | Spain | 50 | 62 | NA | I–IVA | 1 | RT | DFS |
IHC: Immunohistochemistry, LN: Lymph node, LVI: Lymphovascular invasion, LVSI: Lymphovascular space invasion, OS: Overall survival, DFS: Diseasefree survival, RT: Radiotherapy, NA: Not available
| Authors | Method used | Antibody | Pretreatment | Titer | Incubation | Control | Quality |
|---|---|---|---|---|---|---|---|
| Uehara, 1995 | AB complex | DAKO | - | 1:300 | 12 h | Infiltrating lymphocyte | 2 |
| Tjalma, 1997 | AB Complex | DAKO | 0.01 M Citrate microwave | 1:40 | 30 min | Normal lymphocyte | 3 |
| Crawford, 1998 | AB complex | DAKO | Pressure cooker | 1:50 | 40 min | Tonsil | 3 |
| Harima, 1998 | AB complex | DAKO | Microwave | - | - | - | 2 |
| Rajkumar, 1998 | AB complex | DAKO | 0.1 M citrate autoclave | 1:75 | 35 min | Previous known positive sections | 3 |
| Padovan, 2000 | AB complex | DAKO | Microwave | - | Overnight | Normal endometrium | 2 |
| Tjalma, 2001 | Streptavidin | DAKO | 0.01 M citrate buffer, autoclave | 1:40 | 30 min | Tonsil | 4 |
| Chung, 2002 | Streptavidin | Burnham | - | - | - | Previous known positive sections | 2 |
| Grauflund, 2002 | AB complex | DAKO | Microwave, 0.01 M citrate | 1:0 | 30 min | Bladder cancer | 3 |
| Jain, 2003 | AB complex | Novocastra UK | 0.01 M Citrate | 1:80 | - | - | 3 |
| Wootipoom, 2004 | Streptavidin | Novocastra UK | Microwave, 0.01 M citrate | 1:200 | Overnight | Endometrial hyperplasia | 3 |
| Saito, 2004 | AB complex | BD-Biosciences | Microwave, 0.01M citrate | 1:500 | Overnight | - | 2 |
| Munakata, 2005 | - | DAKO | Microwave, 0.01 M citrate | 1:100 | - | Tonsil | 2 |
| Van de Putte, 2005 | Streptavidin | DAKO | Microwave, 0.01 M citrate | 1:20 | 30 min | - | 3 |
| Yamasita, 2009 | Mouse MC | DAKO | Microwave, 1 mM EDTA | 1:80 | 30 min | Lymphoma | 4 |
| Henriquez, 2011 | Streptavidin | DAKO | - | - | 1 h | Positive tumor | 3 |
EDTA: Ethylenediaminetetraacetic acid
Data and analysis evidence synthesis
Overall Survival
A total of 14 studies (n = 1509 patients) reported OS. Positive bcl-2 expression was associated with the decreased RR for OS (lnRR = −0.52; confidence interval: [C.I.] −0.91–−0.13; I2 = 76.89; RR = 0.60 [0.40, 0.88]; P< 0.001). The forest plot and corresponding funnel plot are shown in Figure 1. Six of the included studies showed decreased risk with bcl-2 expression, seven were equivocal, and one study showed expression of bcl-2 to be associated with poor OS. The Egger test showed that there was no evidence of publication bias (P = 0.473) [Figure 2].
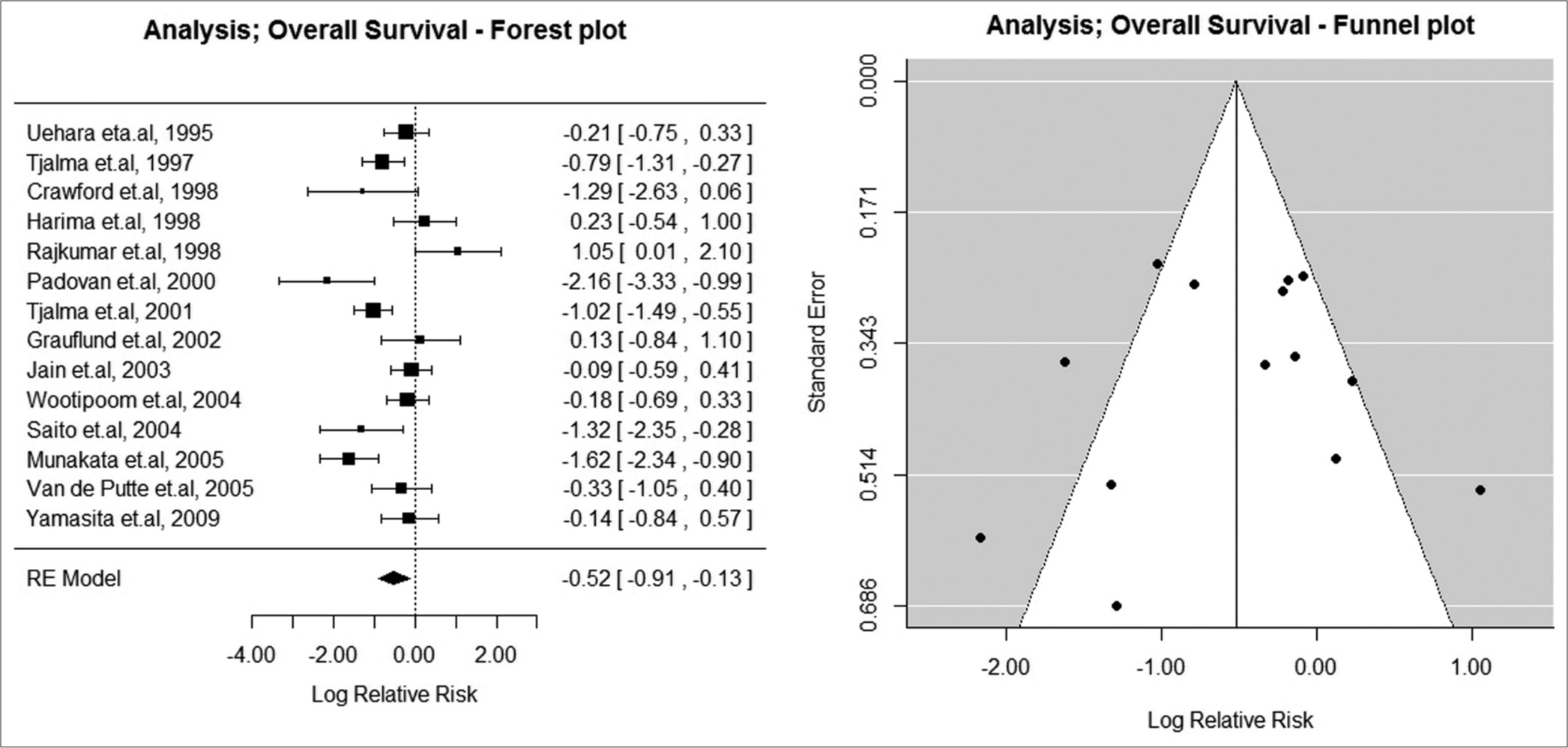
- Overall survival: Forest and funnel plot. Random effects (RE) model (n = 14 studies).
Heterogeneity analysis
We observed three studies contributing to the heterogeneity.[10,21,28] We repeated the meta-analysis by excluding the abovementioned studies. The lnRR was −0.46 (−0.75–−0.18), and there was a significant reduction of heterogeneity (I2 = 46.79). Our results indicate that bcl-2 expression has a potential predictive role for overall survival. There was, however, significant heterogeneity in the meta-analysis for OS. The Egger test showed that there was no evidence of publication bias (P = 0.474).
We investigated the factors contributing to the heterogeneity. We identified the patient, tumor, and treatment-related variables that could have contributed to the heterogeneity. These included stage (early vs. advanced), IHC quality, treatment (radiotherapy ± chemotherapy), and origin (Asian or non-Asian country). The stage-wise distribution of bcl-2 positivity mentioned in the studies is summarized in Table 4. Differences in treatment contributed to the heterogeneity of the study. There was a difference in results between Asian and non-Asian countries, but it was not statistically significant. The IHC method did not significantly contribute to the heterogeneity across the studies.
| Authors (Year) | Bcl-2 positivity and FIGOstage distribution |
|---|---|
| Chung, 2002 | I and II: 25/39 III and IV: 3/4 |
| Crawford, 1998 | N/A |
| Grauflund, 2002 | IA: 18 (24.7%) IB: 48 (65.8%) IIA: 5 (6.9%) IIB: 2 (2.7%) |
| Harima, 1998 | Stage I: 2/27 Stage II: 3/27 Stage III: 15/27 Stage IV: 4/27 |
| Henriquez, 2011 | N/A |
| Jain, 2003 | NA |
| Munakata, 2005 | NA |
| Padovan, 2000 | Stage Ib: 34/39 II: 9/17 III: 9/25 IV: 0/5 |
| Rajkumar, 1998 | Stage IIB: 7 (29.2%) Stage IIIB: 17 (70.8%) |
| Saito, 2004 | I: 6/14 (43%) II: 6/14 (43%) III: 2/14 (14.3%) IV: 0 |
| Tjalma, 1997 | NA |
| Tjalma, 2001 | Ia: 20 (26%) Ib: 24 (32%) IIa: 24 (32%) IIb: 4 (5%) IIIa: 1 (1%) IIIb: 2 (3%) IVa: 1 (1%) IVb: 0 (0%) |
| Uehara, 1995 | Stage Ib: 43/85 (50.5%) Stage IIa: 18/85 (21.17%) Stage IIb: 24/85 (28.23%) |
| Van de Putte, 2005 | Only IB stage included |
| Wootipoom, 2004 | I: 5 (21.7%) II: 13 (13.1%) III and IV: 2 (3.9%) |
| Yamasita, 2009 | Not specified |
| FIGO: Federation Internationale de Gynecologie et d’Obstetrique | |
FIGO: Federation Internationale de Gynecologie et d’Obstetrique
Disease Free Survival
Eight studies described the outcome of DFS.[10,16,18-21,26,29] There was no significant association between the expression of bcl-2 and DFS (lnRR: 0.05 C.I. (−0.30–0.39) [Figure 3].
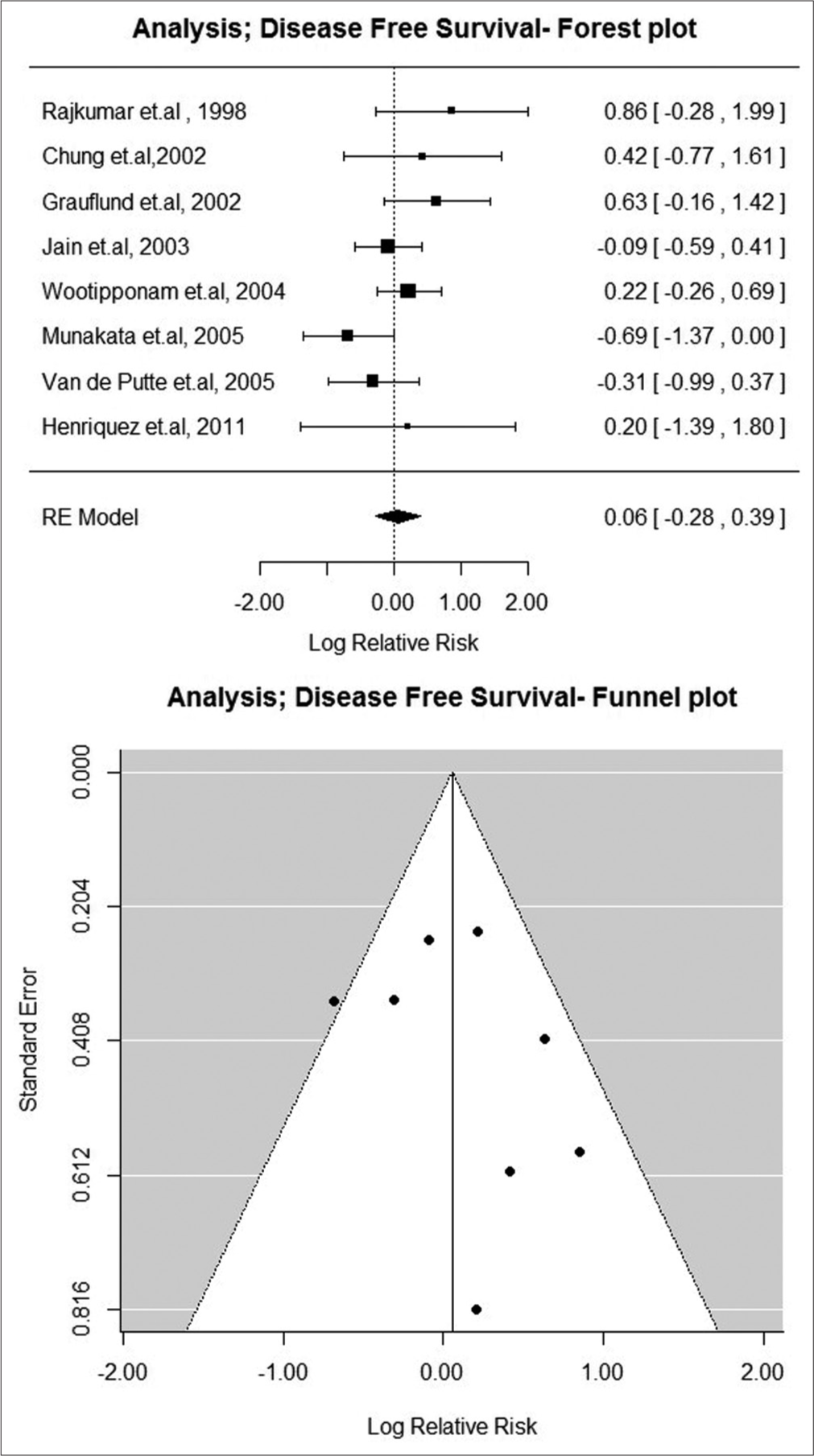
- Disease-free survival: Forest and funnel plot.
DISCUSSION
In locally advanced cervical cancer, the stage is the most important prognostic factor. In addition to the stage, various other patient, tumor, and treatment-related factors can affect the outcome. These factors include the patient’s age, performance status, anemia, lymph node involvement, lymphovascular space invasion, tumor bulk, and hypoxia.[30,31] Although chemoradiation is the current standard of care in locally advanced cervical cancer, the evidence supporting the addition of chemotherapy concurrent with radiotherapy in locally advanced disease, especially in developing countries, has been questioned.[32] Various studies have evaluated the role of the addition of chemotherapy in neo-adjuvant settings.[33,34] In spite of the advancement of radiation therapy and chemotherapy, survival in locally advanced cervical cancer (especially stage III-IV) remains dismal in countries where the disease is highly prevalent.[1] Therefore, the evaluation of prognostic markers is highly relevant in locally advanced cervical cancer.
Apoptosis is one of the important mechanisms of radiation and chemotherapy-induced cell death. Therefore, apoptotic markers have high predictive value in the progression and outcome of various malignancies.[4,35] Apoptosis is evolutionally conserved, highly regulated, non-lytic, programmed cell death. Broadly, it is mediated through intrinsic (or mitochondrial) pathways and extrinsic (or death receptor-mediated) pathways.[36] The bcl-2 family of proteins plays a crucial role in the process of apoptosis and contains one or more bc-2 homology (BH) domains.[37] The family includes proapoptotic proteins (BIM, BID, and PUMA) and anti-apoptotic proteins (BCL-2, BCL-XL), and the apoptotic process is influenced by the balance of these proteins.[38] Activated proapoptotic proteins enable the release of mitochondrial cytochrome c, activating caspase 9 through the formation of apoptosomes.[39]
Although there have been many reports to correlate bcl-2 with outcomes in cervical cancer, the results are still controversial. The difference in results could be due to heterogeneity of the population, IHC technique and threshold value of interpretation, and various treatment modalities.[40] Our systematic review and meta-analysis show that positive bcl-2 expression is associated with better OS. However, there was significant heterogeneity among the studies. Differences in treatment methods significantly contributed to the heterogeneity. We could not conclude any association between DFS and expression of bcl-2.
The expression of bcl-2 was correlated with survival in other solid malignancies, although most of the studies observed significant heterogeneity. Expression of bcl-2 was associated with better survival in lung cancer.[41] A recent lung cancer bcl-2 expression meta-analysis was associated with better overall survival (OS).[42] A meta-analysis of gastric cancer patients found no significant association between bcl-2 and OS. However, subgroup analysis indicated that bcl-2 was associated with better OS in the Asian population.[43]
Breast cancer meta-analysis shows that bcl-2 is a predictive factor for chemotherapy sensitivity.[44] Significant prognostic association of bcl-2 in breast cancer was demonstrated in a meta-analysis done by Callagy et al.[45] A study by Silva et al. showed bcl-2 overexpression associated with the worst prognosis in head-and-neck cancer.[39] However, significant heterogeneity was reported in the study.
The prognostic significance of various biomarkers in cervical cancer has been reviewed in the literature.[2,46] There is a lack of agreement on evidence of the prognostic implications of apoptotic markers. This lacuna in the literature makes the present meta-analysis relevant, despite limitations related to the significant heterogeneity. Bcl-2 was not an independent predictor of response to neoadjuvant chemotherapy in cervical cancer.[47,48] Although there was a difference in bcl-2 staining in CIN and invasive cancer, no significant association with human papillomavirus 16/18 was observed.[49] Bcl-2 expression is increased with the severity of cervical lesions from cervicitis, CIN, and invasive carcinoma.[50]
At present, there is ongoing research on the development of various agents that can target the bcl-2 pathway.[51] These can broadly be classified as antisense oligonucleotide preparations, peptide inhibitors, and small molecule inhibitors of pro-survival BCL-2 proteins.[52] Venetoclax (Bcl-2 selective BH3 analog)–obinutuzumab with or without ibrutinib was superior to chemoimmunotherapy in first-line chronic lymphoid leukemia patients.[53] Therefore, these are promising agents for targeted therapy in the future.
The present study has several limitations. Most of the included studies were retrospective, which is a factor that affects the quality of the evidence. Secondly, in the absence of randomized controlled trials, there is significant heterogeneity as far as the treatment modality, selection of patients, assessment, and interpretation of IHC biomarkers are concerned. However, this result may be helpful in designing more extensive prospective studies encompassing the evaluation of biomarkers as a prognostic factor for disease outcomes.
CONCLUSIONS
bcl-2 can serve as a valuable prognostic factor in cervical cancer, especially for bulky, locally advanced extensive disease in developing countries where treatment outcomes are still poor. Using bcl-2 as a molecular marker may help to prognosticate the disease and intensify the treatment in selected populations. Higher bcl-2 expression was associated with better OS. The same trend was retained in the group of patients treated with surgery. We did not find any association with DFS. Treatment methods significantly contributed to the heterogeneity of the meta-analysis.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Anti-cMet antibody conjugated hollow gold nanospheres as a new nano-material for enhancing the effect of photothermal therapy. Mater Lett. 2015;143:226-9.
- [CrossRef] [Google Scholar]
- Prognostic cell biological markers in cervical cancer patients primarily treated with (chemo)radiation: A systematic review. Int J Radiat Oncol Biol Phys. 2011;79:325-34.
- [CrossRef] [PubMed] [Google Scholar]
- The role of cyclins and cyclins inhibitors in the multistep process of HPV-associated cervical carcinoma. J Egypt Natl Canc Inst. 2006;18:292-302.
- [Google Scholar]
- Anti-apoptosis and cell survival: A review. Biochim Biophys Acta. 2011;1813:238-59.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of Bcl-2 in breast cancer patients treated with neoadjuvant anthracycline based chemotherapy. Mol Oncol. 2008;2:102-11.
- [CrossRef] [PubMed] [Google Scholar]
- BCL-2: A new therapeutic target in estrogen receptor-positive breast cancer? Cancer Cell. 2013;24:7-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia and expression of the proapoptotic regulator BNIP3 in cervical cancer. Int J Gynecol Cancer. 2006;16:1314-20.
- [CrossRef] [PubMed] [Google Scholar]
- The role of p53, Bcl-2 and Ki-67 in premalignant cervical lesions and cervical cancer. Eur J Gynaecol Oncol. 2007;28:290-3.
- [Google Scholar]
- Molecular profiling of cervical cancer progression. Br J Cancer. 2007;96:321-8.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of Fas ligand and bcl-2 in cervical carcinoma and their prognostic significance. Am J Clin Pathol. 2005;123:879-85.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical potential of inhibitors of survival pathways and activators of apoptotic pathways in treatment of cervical cancer: Changing the apoptotic balance. Lancet Oncol. 2005;6:589-98.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of procyanidins from Pinus koraiensisbark on growth inhibition and expression of PCNA and TNF-a in mice with U14 cervical cancer. Therapy. 2007;4:685-90.
- [CrossRef] [Google Scholar]
- Bax and Bcl-2 expression predict response to radiotherapy in human cervical cancer. Gan To Kagaku Ryoho. 1998;25:1273-7.
- [CrossRef] [Google Scholar]
- Molecular predictive factors of outcome of radiotherapy in cervical cancer. Neoplasma. 2011;58:469-75.
- [CrossRef] [PubMed] [Google Scholar]
- Preferred reporting items for systematic review and metaanalysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of apoptotic regulators and their significance in cervical cancer. Cancer Lett. 2002;180:63-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of the bcl-2 apoptotic family of proteins in primary and recurrent cervical cancer. Br J Cancer. 1998;78:210-4.
- [CrossRef] [PubMed] [Google Scholar]
- MIB-1, p53, bcl-2, and WAF-1 expression in pelvic lymph nodes and primary tumors in early stage cervical carcinomas: Correlation with clinical outcome. Int J Oncol. 2002;20:1041-7.
- [CrossRef] [PubMed] [Google Scholar]
- BCL-2, in combination with MVP and IGF-1R expression, improves prediction of clinical outcome in complete response cervical carcinoma patients treated by radiochemotherapy. Gynecol Oncol. 2011;122:585-9.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of p53 and Bcl-2 expression as prognostic markers in invasive cervical carcinoma stage IIb/III patients treated by radiotherapy. Gynecol Oncol. 2003;88:22-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of Bcl-2 and p53 protein expression in stage IIB and IIIB squamous cell carcinoma of the cervix. Eur J Gynaecol Oncol. 1998;19:556-60.
- [Google Scholar]
- Correlation between responsiveness of neoadjuvant chemotherapy and apoptosis-associated proteins for cervical adenocarcinoma. Gynecol Oncol. 2004;92:284-92.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of bcl-2 expression in patients with operable carcinoma of the uterine cervix. J Clin Pathol. 1997;50:33-6.
- [CrossRef] [PubMed] [Google Scholar]
- The importance of biological factors (bcl-2, bax, p53, PCNA, MI, HPV and angiogenesis) in invasive cervical cancer. Eur J Obstet Gynecol Reprod Biol. 2001;97:223-30.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of the proto-oncogene bcl-2 in uterine cervical squamous cell carcinoma: Its relationship to clinical outcome. Eur J Gynaecol Oncol. 1995;16:453-60.
- [Google Scholar]
- Prognostic significance of Bax, Bcl-2, and p53 expressions in cervical squamous cell carcinoma treated by radiotherapy. Gynecol Oncol. 2004;94:636-42.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation among six biologic factors (p53, p21(WAF1), MIB-1, EGFR, HER2, and Bcl-2) and clinical outcomes after curative chemoradiation therapy in squamous cell cervical cancer. Int J Radiat Oncol Biol Phys. 2009;74:1165-72.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of bcl-2, p53 and Ki-67 in invasive squamous carcinoma of the uterine cervix. Eur J Gynaecol Oncol. 2000;21:267-72.
- [Google Scholar]
- Markers of apoptosis in stage IB squamous cervical carcinoma. J Clin Pathol. 2005;58:590-4.
- [CrossRef] [PubMed] [Google Scholar]
- Relation between HPV-DNA and expression of p53, bcl-2, p21WAF-1, MIB-1, HER-2/neu and DNA ploidy in early cervical carcinoma: Correlation with clinical outcome. Oncol Rep. 2004;12:169-76.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of Bcl-2 in dysplastic and neoplastic cervical lesions in relation to cell proliferation and HPV infection. Neoplasma. 2000;47:143-7.
- [Google Scholar]
- Does the evidence support the use of concurrent chemoradiotherapy as a standard in the management of locally advanced cancer of the cervix, especially in developing countries? Clin Oncol (R Coll Radiol). 2006;18:306-12.
- [CrossRef] [PubMed] [Google Scholar]
- Low-dose fractionated radiation and chemotherapy prior to definitive chemoradiation in locally advanced carcinoma of the uterine cervix: Results of a prospective phase II clinical trial. Gynecol Oncol. 2015;138:292-8.
- [CrossRef] [PubMed] [Google Scholar]
- LBA8 A randomised phase III trial of induction chemotherapy followed by chemoradiation compared with chemoradiation alone in locally advanced cervical cancer: The GCIG INTERLACE trial. Ann Oncol. 2023;34:S1276.
- [CrossRef] [Google Scholar]
- Prognostic value of cell proliferation and apoptosis in uterine cervical cancer treated with radiation. Zhonghua Zhong Liu Za Zhi. 1999;21:290-2.
- [Google Scholar]
- Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495-516.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20:175-93.
- [CrossRef] [PubMed] [Google Scholar]
- BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;10:177.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of Bcl-2 expression with prognosis and survival in patients with head and neck cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2023;187:104021.
- [CrossRef] [PubMed] [Google Scholar]
- Association between Bcl-2 expression and tumor recurrence in cervical cancer: A matched casecontrol study. Gynecol Oncol. 2006;102:263-9.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of bcl-2 protein in non-small cell lung cancer: Correlation with clinicopathology and patient survival. Neoplasma. 1999;46:25-30.
- [Google Scholar]
- High expression of Bcl-2 protein predicts favorable outcome in non-small cell lung cancer: Evidence from a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:8861-9.
- [CrossRef] [PubMed] [Google Scholar]
- Bcl-2 expression and patient survival in gastric cancer: A systematic review of the literature with meta-analysis. Med Oncol. 2015;32:389.
- [CrossRef] [PubMed] [Google Scholar]
- Bcl-2 expression predicts sensitivity to chemotherapy in breast cancer: A systematic review and meta-analysis. J Exp Clin Cancer Res. 2013;32:105.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer. 2008;8:153.
- [CrossRef] [PubMed] [Google Scholar]
- Cervical Carcinoma: Oncobiology and biomarkers. Int J Mol Sci. 2021;22:12571.
- [CrossRef] [PubMed] [Google Scholar]
- Are biomarkers expression and clinical-pathological factors predictive markers of the efficacy of neoadjuvant chemotherapy for locally advanced cervical cancer? Eur J Surg Oncol. 2024;50:108311.
- [CrossRef] [PubMed] [Google Scholar]
- CD34 and Bcl-2 as predictors for the efficacy of neoadjuvant chemotherapy in cervical cancer. Arch Gynecol Obstet. 2021;304:495-501.
- [CrossRef] [PubMed] [Google Scholar]
- p53 and bcl2 expression in malignant and premalignant lesions of uterine cervix and their correlation with human papilloma virus 16 and 18. South Asian J Cancer. 2014;3:48-53.
- [CrossRef] [PubMed] [Google Scholar]
- Expression levels of survivin, Bcl-2, and KAI1 proteins in cervical cancer and their correlation with metastasis. Genet Mol Res. 2015;14:17059-67.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018;8:180002.
- [CrossRef] [PubMed] [Google Scholar]
- The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front Oncol. 2022;12:985363.
- [CrossRef] [PubMed] [Google Scholar]
- First-line venetoclax combinations in chronic lymphocytic leukemia. N Engl J Med. 2023;388:1739-54.
- [CrossRef] [PubMed] [Google Scholar]






