Translate this page into:
Prostatic perivascular epithelioid cell tumor: A rare entity and literature review
*Corresponding author: Nisha Modi, Department of Pathology, Sri Aurobindo Medical College and Post Graduate Institute, Indore, Madhya Pradesh, India. nishamodib@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Modi N, Gundawar RP, Ayachit RS. Prostatic perivascular epithelioid cell tumor: A rare entity and literature review. J Lab Physicians. 2024;16:210-4. doi: 10.25259/JLP-16-210-(1941)
Abstract
Perivascular epithelioid cell tumor (PEComa) is a rare mesenchymal neoplasm comprising perivascular epithelioid cells that express myomelanocytic immunophenotype, while stain negative for epithelial markers. We describe a case of prostatic PEComa in a 55-year-old man, who had one month history of frequent urination, hesitancy, and sensation of incomplete voiding. Radiological imaging disclosed prostatomegaly. Despite the medical treatment offered, episodes of urinary retention persisted. Subsequently, he underwent endoscopic transurethral resection of the prostate, histology showed tumor arranged in nests surrounded by thin delicate vessels. The tumor cells were epithelioid with abundant clear-to-eosinophilic cytoplasm, round nuclei, and inconspicuous nucleoli. Bizarre multinucleated giant cells, conspicuous mitosis and focal necrosis were evident. On immunohistochemistry, the tumor cells were diffusely positive for Human Melanoma Black (HMB45) and smooth muscle actin, negative for anti-cytokeratin monoclonal antibodies (AE1/AE3), Homeobox protein (NKX3.1), GATA Binding Protein 3 (GATA3), synaptophysin, Transcription Factor Binding to Immunoglobulin Heavy Constant Mu (IGHM) Enhancer 3 (TFE3), desmin, and SRY (sex determining region Y) -Box Transcription Factor 10 (SOX10). This uncommon case illustrates our diagnostic ordeal with a brief discussion on its nosology and a comprehensive literature review.
Keywords
Perivascular epithelioid cell tumor
PEComa
Prostate
HMB45
Smooth muscle actin
Myomelanocytic tumor
Folpe criteria
INTRODUCTION
According to the World Health Organization (WHO) classification of soft-tissue tumors, perivascular epithelioid cell tumors (PEComas) comprise a group of rare mesenchymal neoplasms that arise from a morphologically and immunophenotypically distinct perivascular epithelioid cell (PEC) which is an undifferentiated cell of neural crest origin. These include angiomyolipomas of the kidney and liver, clear cell “sugar” tumors of the lung, lymphangioleiomyomatosis, along with a group of similar lesions arising at a variety of visceral and soft-tissue sites.[1] PEComas are characterized by a unique epithelioid cell type and coexpression of smooth muscle and melanocytic immunohistochemical markers. A strong association has been demonstrated between PEComas and the tuberous sclerosis complex (TSC).[2] Furthermore, a subset of PEComas is also associated with transcription factor E3 (TFE3) gene rearrangements, which happen to be mutually exclusive to those associated with TSC.[2] Although ubiquitous, PEComa is an extremely rare clinicopathological entity in the prostate with limited documentation. Due to many unsettled issues regarding the diagnostic criteria, pathogenesis, and treatment, further addition of details of individual cases remains quintessential. This case exemplifies the diagnostic challenges associated with it that necessitates the application of diverse immunohistochemistry (IHC) markers for its correct identification, along with a review of the nosological position of PEComas as they may have an aggressive clinical course, including distant metastases.
CASE REPORT
A 55-year-old male had one month history of frequent urination, hesitancy, and sensation of incomplete voiding. No family history or personal stigmata of TSC was found. His general physical examination, hemogram, biochemical, urine analysis and serum levels of prostate-specific antigen (PSA = 4.2 ng/mL), and carcinoembryonic antigen were within normal limits. Digital rectal examination revealed uniformly enlarged, non-tender, and rubbery prostate. Thereafter, he underwent radiological evaluation. Pelvic ultrasonography disclosed grade 2 prostatomegaly with loss of the median groove. A month later, despite the medical treatment provided, his episodes of urinary retention kept recurring. Subsequently, the patient underwent transurethral resection of the prostate (TURP), which was reported as benign prostatic hyperplasia elsewhere. His complaints pertaining to intermittent prostatomegaly continued, for which a repeat TURP was performed and was submitted to us for histopathological evaluation. On microscopic examination, a tumor was seen displaying nests and sheets of uniform epithelioid cells with perivascular arrangement [Figure 1a and b]. The tumor cells showed moderate nuclear atypia, inconspicuous nucleoli, abundantly clear to eosinophilic cytoplasm, and distinct cell borders [Figure 1c and d]. Remarkably bizarre multinucleated giant cells along with conspicuous mitosis and focal necrosis were observed [Figure 1b-d]. Based on these histological features and location, differential diagnosis considered were malignant melanoma (MM), clear cell sarcoma (CCS), clear cell carcinoma (CCC), paraganglioma, metastatic carcinoma, and prostatic carcinoma and an immunohistochemical panel was performed for this purpose. On IHC, the tumor cells were positive for Human Melanoma Black (HMB45) [Figure 2a], while it was negative for anti-cytokeratin monoclonal antibodies (AE1/AE3)AE1/AE3 [Figure 2b]. Smooth muscle actin (SMA) [Figure 2c] was diffusely expressed in the tumor cells, while were immunonegative for SRY (sex determining region Y) -Box Transcription Factor 10 (SOX10) [Figure 2d], Homeobox protein (NKX3.1), GATA Binding Protein 3 (GATA3), synaptophysin, desmin and TFE3. Considering the aforementioned histopathological and immunohistochemical findings, diagnosis of malignant PEComa of prostate was rendered. An accompanying conventional prostatic adenocarcinoma was not observed. Thorough imaging studies revealed that there was no evidence of any lesion elsewhere in the patient. Later on, he was offered radical prostatectomy and adjuvant chemotherapy, doxorubicin, and cisplatin + mammalian target of rapamycin inhibitors courses and was put on follow-up (FU). There is no recurrence yet, and the patient has been clinically free of disease 26 months post-treatment. Routine FU procedure includes periodic rectal examination, PSA screening, and computed tomography of the pelvis.
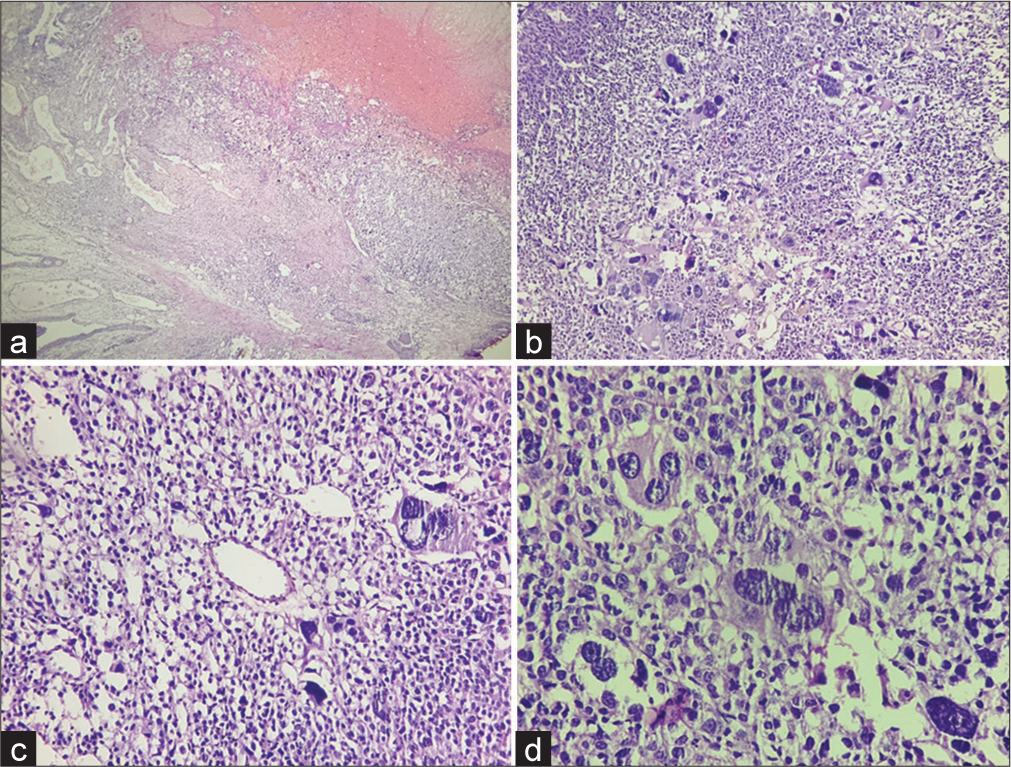
- Histopathological findings (a) A low-power view shows nested architecture of the tumor at the right upper and the epithelium of prostatic urethra at the left lower corner (Hematoxylin and eosin, ×4). (b) Many bizarre multinucleated giant cells are seen (Hematoxylin and eosin, ×10). (c and d) Photomicrograph showing tumor cells surrounded by thin delicate vessel and are epithelioid with abundant clear-to-eosinophilic cytoplasm, round nuclei and prominent nucleoli (Hematoxylin and eosin, ×20 and ×40 respectively).
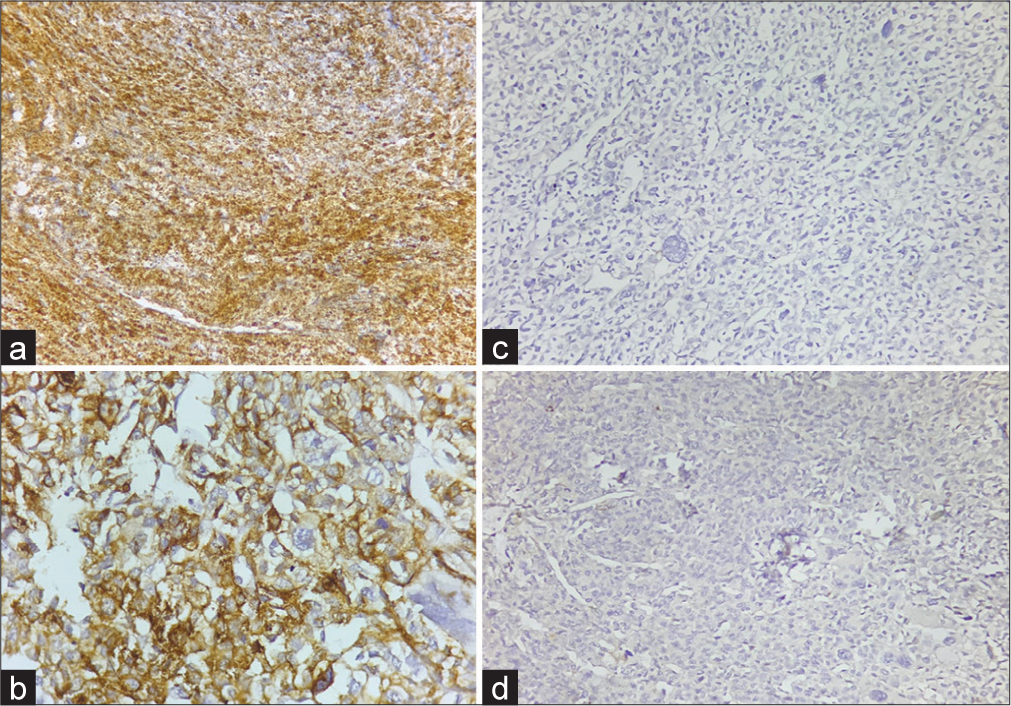
- (a) Diffuse Human Melanoma Black 45 positive tumor cells (Diaminobenzidine, ×20). (b) Immunohistochemistry (IHC) showing absent staining for Anti-cytokeratin monoclonal antibodies (Diaminobenzidine, ×20). (c) Diffuse positivity for smooth muscle actin in tumor cells (Diaminobenzidine, ×40). (d) IHC showing absent staining for SRY (sex determining region Y) -Box transcription factor 10 (Diaminobenzidine, ×20).
DISCUSSION
Bonetti et al. coined the term PEC and PEComa was introduced by Zamboni with regard to epithelioid lesions with clear-to-eosinophilic cytoplasm and a perivascular distribution.[3] PEComa so far has remained an umbrella term for a group of mesenchymal tumors showing epithelioid and spindled clear cells in a characteristic trabecular arrangement. Nonetheless, PEComas are now widely accepted as a well-defined class of stromal cell tumors by the WHO, with defined essential diagnostic criteria which state the presence of mixed epithelioid and spindle cell morphology in variable proportions, granular eosinophilic to clear cytoplasm, nested/trabecular/sheet-like architecture with frequent perivascular orientation, and coexpression of various melanoma associated and smooth muscle antigens with a behavioral spectrum from being benign to frankly malignant.[1] Desirable diagnostic criteria include TFE3 expression if smooth muscle markers are negative. For cases with strong TFE3 protein expression, identification of TFE3 gene rearrangement helps in confirming the diagnosis.[1] Prostatic PEComas are rare and only three cases have been currently described. We have reported the literature review of prostatic PEComa in Table 1.[4-7]
| Pan et al.[4] | Eken and Saglican[5] | Sbrollini et al.[7] | Wang et al.[6] | Present case | |
|---|---|---|---|---|---|
| Year | 2003 | 2014 | 2014 | 2016 | 2021 |
| Diagnosis | Malignant PEComa | Benign PEComa | Malignant PEComa | Primary Xp11 neoplasm with melanocytic differentiation | Malignant PEComa |
| Age/sex (years) | 46/M | 36/M | 54/M | 42/M | 55/M |
| Symptoms | Frequent urination, hesitancy, dribbling | Intermittent blood tinged Ejaculates | Acute urinary retention, dysuria, nocturia | Dysuria, hesitancy, dribbling | Frequent urination, hesitancy, sensation of incomplete void |
| Specimen | Radical prostatectomy | Laser prostatectomy | TURP | TURP | TURP |
| Size (cm) | 8.5×6.5×5.5 | NA | NA | NA | NA |
| Cell type | Epithelioid | Epithelioid and spindle | Epithelioid and spindle | Epithelioid | Epithelioid |
| Border | Well-delimited | Infiltrative | Infiltrative | Infiltrative | Well-delimited |
| Nuclear atypia | Mild to moderate | Absent | Marked | Mild to moderate | Moderate |
| Mitosis/50 hpf | Low | Absent | High | Low | High |
| Necrosis | Focal | Absent | Present | Focal | Focal |
| Vascular invasion | Absent | Absent | Absent | Present | Absent |
| Immunopositivity | HMB45, NSE | HMB45 | HMB45, TFE3, Melan-A, Desmin, caldesmon, calponin, SMA, vimentin | HMB45, TFE3, cathepsin K, P504S | HMB45, SMA |
| Immunonegativity | AE1/AE3, CAM 5.2, 34bE-12, MNF-116, KL-1, EMA, S100, Melan-A, vimentin, HHF35, SMA, PSA, PAP, CEA, B72.3 | Melan-A, S100, SMA, CD68, synaptophysin, chromogranin-A, AE1/AE3, PSA, vimentin | - | Melan-A, SMA, PSA, PSMA, AE1/AE3, S100, PAX8, Ki-67–10% | AE1/AE3, NKX3.1, GATA3, Synaptophysin, Desmin, TFE3, SOX10 |
| Treatment | Surgery+adjuvant CT | Surgery | Surgery+adjuvant CT+Pazopanib | Surgery | Surgery+adjuvant CT |
| FU (months) | 48 | 30 | 6 | 5 | 26 |
| Outcome | Lung metastases at 3 years; DOD at 4 years | NED | Lung metastases at the presentation | NED | NED |
| Status | Dead | Alive | Alive | Alive | Alive |
PEComa: Perivascular epithelioid cell tumor, TURP: Transurethral resection of prostate, M: Male, NA: Not available, SMA: Smooth muscle actin, PSMA: Prostate-specific membrane antigen, CEA: Carcinoembryonic antigen, NSE: Neuron-specific enolase, EMA: Epithelial membrane antigen, FU: Follow-up, PAP: Prostatic acid phosphatase, CT: Chemotherapy, NED: No evidence of disease, DOD: Dead of disease, TFE3: Transcription factor E3, PSA: Prostate-specific antigen, CAM 5.2: Anti-cytokeratin monoclonal antibody, 34bE-12: Cytokeratin 34 beta E12; MNF-116: Anti-cytokeratin antibody, KL-1: Anti-cytokeratin antibody, EMA: Epithelial membrane antigen, S100: S100 protein, CD68: Cluster of differentiation 68, PAX8: Paired box gene-8, Ki: Ki monoclonal antibody, HMB45: Human Melanoma Black, AE1/AE3: Anti-cytokeratin monoclonal antibodies, NKX3.1: Homeobox protein, GATA3: GATA Binding Protein 3, SOX10: (sex determining region Y) box 10 protein, B72.3: Tumor associated glycoprotein 72 antibody, HF35: Actin, muscle specific mouse monoclonal antibody, Melan-A: Melanoma antigen.
When PEComa originates from the fibromuscular stroma of the prostate, it mimics benign prostatic enlargement and causes lower urinary tract symptoms. In the present case, the histopathological features of epithelioid tumor cells with clear to eosinophilic cytoplasm, organoid pattern of arrangement, characteristically around blood vessels, led to consideration of differential diagnoses including MM, CCS, CCC, paraganglioma, metastatic carcinoma, and a remote possibility of prostatic carcinoma. Absence of prominent nucleoli lowered our suspicion of MM. Due to its myomelanocytic immunophenotype, PEComa can easily be confused with MM, but our case was negative for SOX10 which is a known sensitive and specific marker of MM. Lack of discrete solid mass in prostate and IHC negativity for AE1/AE3, Homeobox protein (NKX3.1) ruled out the possibility of prostate carcinoma. The aforementioned IHC profile similarly ruled out the considerations of metastatic carcinoma and CCC. The present tumor was unlikely to be paraganglioma, given its complete absence of expression of GATA3 and synaptophysin. HMB45 positivity with coexpression of SMA led to diagnosis of PEComa in this case. Therefore, IHC was necessary for an objective diagnosis of this rare entity. A novel translocation that occurs between the NONO (p54nrb) and TFE3 genes has been observed in one of the documented prostatic PEComa.[6] NONO (p54nrb) plays an important role in disease progression and metastasis. Histopathologic features described in TFE3-translocated prostatic PEComa are epithelioid morphology, infiltrative pattern, presence of minor atypia with mitosis, necrosis, and lymphovascular invasion (LVI). Thus, our case was also evaluated for TFE3 by IHC which was negative.
Various histologic criteria have been proposed till now for assessing the malignant potential of PEComa. Unfortunately, given their rarity, reliable criteria have yet to be established. The first and most widely used criteria for predicting clinical behavior of all anatomic sites was proposed by Folpe et al. in 2005.[8] It stratified PEComas into three prognostic categories : benign, uncertain malignant potential (UMP), and malignant, based on evaluation of six worrisome histopathologic features (tumor size >5 cm, high-grade nuclear atypia, mitotic activity, necrosis, LVI, and infiltrative border). Subsequently, certain deficiencies in this system became obvious. While the categorization of cases with no worrisome features (benign) or two or more worrisome features (malignant) was forthright, it was ambiguous how to classify those PEComas with a single worrisome feature such as high mitotic count or necrosis. Therefore, in 2012, a revised version of Folpe criteria was proposed by Bleeker et al.[9] They included only two histological features : tumor size and mitotic activity, and showed better specificity in categorizing PEComa with aggressive behavior as “malignant” compared to the original Folpe criteria. Later on, in 2015, Conlon et al. proposed another criteria applicable to gynecologic PEComas, which they called Modified Folpe criteria.[10] They retained the three-tier stratification (benign, UMP, and malignant) and utilized either necrosis or two or more of the six original worrisome features for the prediction of malignant behavior and less than one worrisome feature for the prediction of benign behavior. Testing above algorithms in prostatic PEComas in future studies with long FU are needed to verify the effectiveness of these prognostic classification and provide better accuracy and higher applicability. At present, the WHO classification of soft-tissue tumors does not enlist any particular criteria or specific recommendation for use of either of the systems described above.[1] However, they specify that malignant tumors are typically large and show marked nuclear atypia and pleomorphism, conspicuous mitoses, necrosis, and infiltrative margins, and tend to seek an aggressive clinical behavior. Tumor size >5 cm has also been significantly associated with recurrence. The most common metastatic sites are the liver, lymph nodes, lungs, and bone.[1] Malignant PEComa can be very aggressive, leading to multiple metastases and death. Consequently, we concur that all the cases of PEComa, at the current time , must be prognostically stratified. Our case was evidently malignant according to the malignant criteria described by Folpe et al.[8] We recommend that this kind of tumor must be recognized and confirmed by an appropriate IHC study when presented with a clear cell epithelioid neoplasm and perivascular arrangement.
CONCLUSIONS
In conclusion, we have explained the development of the concept of prognostic stratification of PEComa family highlighted what is known about prostatic PEComa at an histomorphological, immunohistochemical, and molecular level, and presented a summary of all the reported cases till date, and briefly discussed our dilemma in establishing the correct diagnosis of this unusual tumor.
Ethical Approval
The research/study complied with the Helsinki Declaration of 1964.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- World health organization classification of tumours of soft tissue and bone In: PEComa (5th ed). Lyon, France: IARC Press; 2020. p. :312-4.
- [Google Scholar]
- TFE3 gene rearrangement in perivascular epithelioid cell neoplasm (PEComa) of the genitourinary tract. Clin Genitourin Cancer. 2020;18:e692-7.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant perivascular epithelioid cell tumor involving the prostate. Arch Pathol Lab Med. 2003;127:E96-8.
- [CrossRef] [PubMed] [Google Scholar]
- Primary perivascular epithelioid cell tumour (PEComa) of the prostate. Can Urol Assoc J. 2014;8:E455-7.
- [CrossRef] [PubMed] [Google Scholar]
- Xp11 neoplasm with melanocytic differentiation of the prostate harbouring the novel NONO-TFE3 gene fusion: Report of a unique case expanding the gene fusion spectrum. Histopathology. 2016;69:450-8.
- [CrossRef] [PubMed] [Google Scholar]
- Perivascular epithelioid cell tumor (PEC-ome) of the prostate: Ultrasound feature in case report. Arch Ital Urol Androl. 2014;86:393-4.
- [CrossRef] [PubMed] [Google Scholar]
- Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: A clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558-75.
- [CrossRef] [PubMed] [Google Scholar]
- "Malignant" perivascular epithelioid cell neoplasm: Risk stratification and treatment strategies. Sarcoma. 2012;2012:541626.
- [CrossRef] [PubMed] [Google Scholar]
- Perivascular epithelioid tumours (PEComas) of the gynaecological tract. J Clin Pathol. 2015;68:418-26.
- [CrossRef] [PubMed] [Google Scholar]






