Translate this page into:
Rare presentation of mixed autoimmune hemolytic anemia in children: Report of 2 cases
Address for correspondence: Dr. Deeksha Singh, C1/04, Mangal Apartments, Vasundhara Enclave, New Delhi - 110 096, India. E-mail: deepshikha.singh293@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Immune hemolytic anemia is characterized by clinical and laboratory features of hemolytic anemia with direct antiglobulin test (DAT) positivity. It could be autoimmune hemolytic anemia (AIHA), alloimmune, or drug-induced hemolysis based on the antigenic stimulus. Furthermore, based on thermal amplitude of autoantibody, AIHA is classified as warm (65%), cold (30%), and mixed (5%) type. Mixed AIHA is extremely rare in children and must be differentiated from warm AIHA with clinically insignificant cold agglutinins and cold hemagglutinin disease as their treatment is different. It may present as blood group discrepancy or cross-match incompatibility leading to delay in arranging suitable blood unit for transfusion. Therefore, a thorough immunohematology workup including monospecific DAT, indirect antiglobulin test at 4°C and 37°C, determination of thermal amplitude and titer is essential. We hereby present two pediatric cases of mixed AIHA presenting as ABO forward and reverse blood group discrepancy and cross-match incompatibility.
Keywords
Autoantibody
autoimmune hemolytic anemia
best match blood
direct antiglobulin test
transfusion
Introduction
Immune hemolytic anemia is defined as shortened red blood cells (RBC) survival mediated through immune response specifically by humoral antibody. It presents with anemia, jaundice, and splenomegaly with reticulocytosis, unconjugated hyperbilirubinemia, and direct antiglobulin test (DAT) positivity.[1] It could be autoimmune (AIHA), alloimmune, or drug-induced hemolysis based on the antigenic stimulus.[2] Furthermore, based on the thermal amplitude of autoantibody, AIHA is classified as warm (65%), cold (30%), and mixed (5%). On the basis of underlying etiology, AIHA can be primary/idiopathic (50%) or secondary (50%).[3] AIHA shows female preponderance (male:female = 1:1.5) and is uncommon in young patients affecting approximately 0.2/100,000 in 11–20 years age group.[145] Out of these, <5% are mixed AIHA.[6]
We hereby present two cases of mixed AIHA presenting as ABO forward and reverse blood group discrepancy and cross-match incompatibility.
Case Report
Case 1 was a 17-year-old girl with pallor, abdominal pain, and yellowish discoloration of eyes for 5 days while case 2 was a 6-year-old female child with pallor, yellowish discoloration of eyes for 1 month and passage of red-colored urine 5 days back. Physical examination of both cases revealed pallor, icterus, and splenomegaly. No lymphadenopathy, rash, or acrocyanosis were noted. There was no history of blood transfusion or significant medical history in case 1. However, there was a history of transfusion of 3 units packed RBCs (PRBCs) 1 month back in case 2. In both cases, complete blood count showed similar findings with severe anemia, raised mean corpuscular volume, mean corpuscular hemoglobin (MCH) and MCH concentration, reduced RBC count, and raised corrected reticulocyte count. Rest of the automated cell counts (total leukocyte count, differential leukocyte count, and platelet count) were within normal limits. Peripheral smear showed marked agglutination of RBCs with many polychromatophils and nucleated RBCs. No abnormal cell or malarial parasite was seen. Serum indirect bilirubin and lactate dehydrogenase were raised. Other biochemical parameters were within normal limits. Serum for viral markers and antinuclear antibody was negative. Urinalysis for hemoglobinuria was within normal limits in both cases. Pearl's reaction for hemosiderin could not be done in case 2 [Table 1].
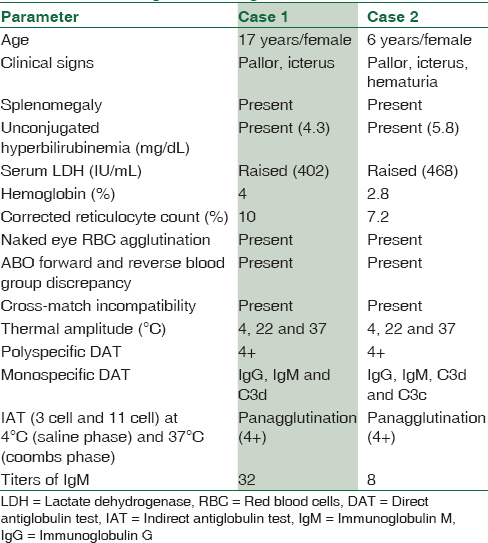
Laboratory investigations
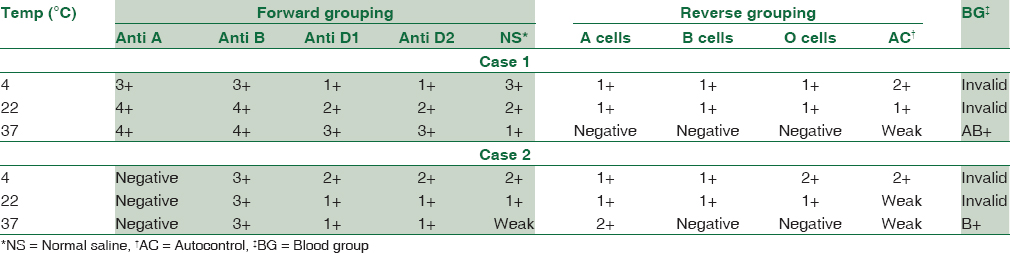
In view of severe anemia, urgent requisition for PRBCs was received for both cases in our Regional Blood Transfusion Centre (RBTC). Both the cases showed naked eye RBC agglutination of the blood sample with discrepancy in forward and reverse blood grouping at room temperature and cross-match incompatibility. Therefore, the patient's fresh EDTA sample was collected under strict warm conditions. The RBCs and serum were separated immediately by centrifugation at 2000 rpm for 5 min. The cells were washed multiple times with warm normal saline. In both cases, extended forward and reverse blood grouping was done at 4°C, 22°C, and 37°C by tube method which showed discrepant results at 4°C and 22°C. However, at 37°C, the blood group was confirmed as AB Rh positive (case 1) and B Rh positive (case 2), respectively. In both cases, autocontrol were positive at 4°C, 22°C, and 37°C, suggesting the presence of autoantibody [Table 2].
Polyspecific DAT (Immunoglobulin G [IgG] and C3d) was strongly positive (4+) in both cases. Monospecific DAT revealed strong reaction (4+) with IgG, immunoglobulin M (IgM), and C3d in both cases while case 2 showed reaction with C3c also [Figure 1]. Antibody screening by three-cell panel (ID-DiaCell I-II-III Asia, Diamed, Switzerland) and antibody identification by extended 11-cell panel (DiaMed 11 cell ID-DiaPanel) showed panagglutination of uniform strength at 37°C (in Coomb's phase) and at 4°C (in saline phase) in both cases, suggestive of autoantibody [Figure 2a and b]. The Rh/Kell/extended antigen profile by using column agglutination technology (DiaClon gel card, Diamed Switzerland) was done for both patients [Figure 3]. The IgM antibody titer done by double dilution tube method in saline phase at 4°C was 32 in case 1 and 8 in case 2, respectively. Thus, based on the above findings, the possibility of both warm (IgG) and cold (IgM) autoantibodies with high thermal amplitude and low titer, i.e., mixed AIHA was suggested in both cases [Figure 4]. Least incompatible, ABO and Rh/Kell-matched, fresh prewarmed PRBCs were slowly transfused in warm environment to the respective patients under strict clinical supervision and steroid cover. Transfusion was uneventful. Both patients were started on corticosteroid therapy and discharged in clinically stable condition. However, the patients were lost to follow-up thereafter.
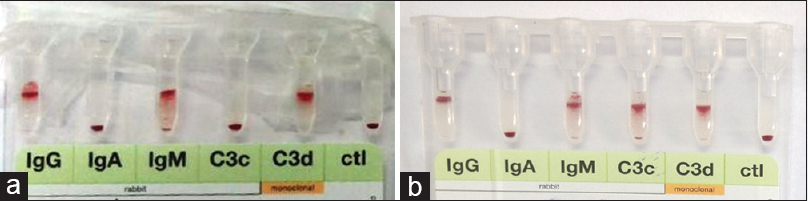
- Monospecific direct antiglobulin test by column agglutination technology. (a) Case 1 – positive for IgG, IgM, and C3d (b) Case 2 – positive for IgG, IgM, C3c, and C3d
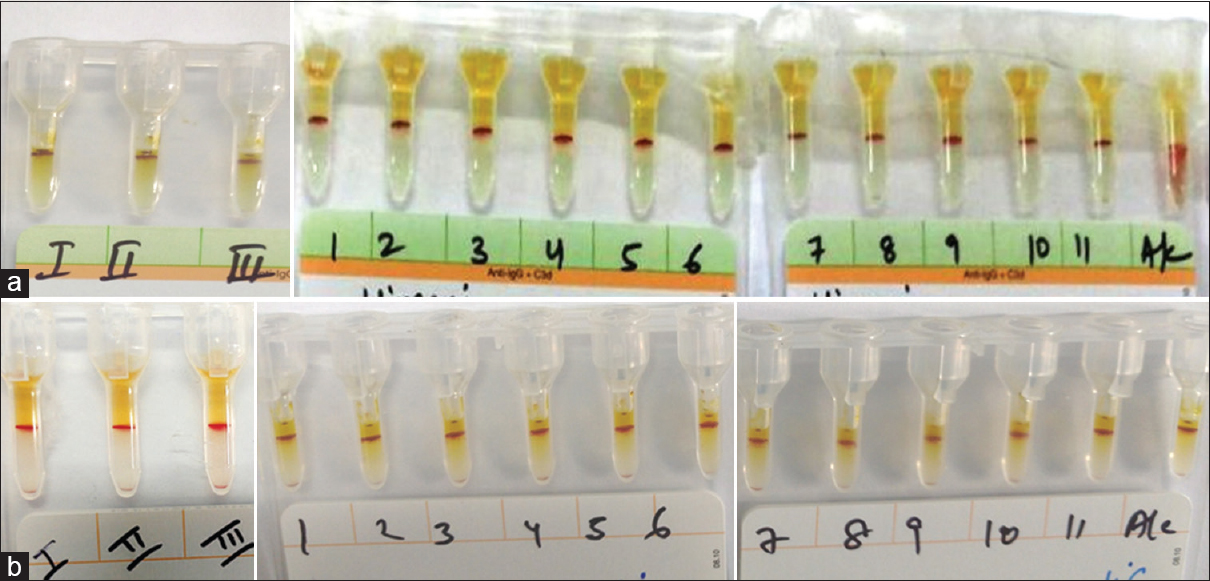
- (a) Indirect antiglobulin test showing panagglutination (4+) in case 1 using antibody screening 3-cell panel and antibody identification 11-cell panel at 37°C. (b) Indirect antiglobulin test showing panagglutination (4+) in case 1 using antibody screening 3-cell panel and antibody identification 11-cell panel at 4°C
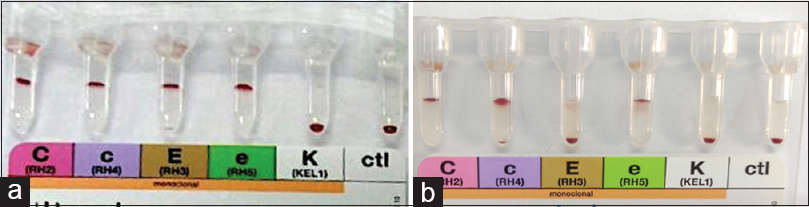
- Rh/Kell antigen profile (a) case 1 positive for C, c, E and e antigens (b) case 2 positive for C, c and e antigens
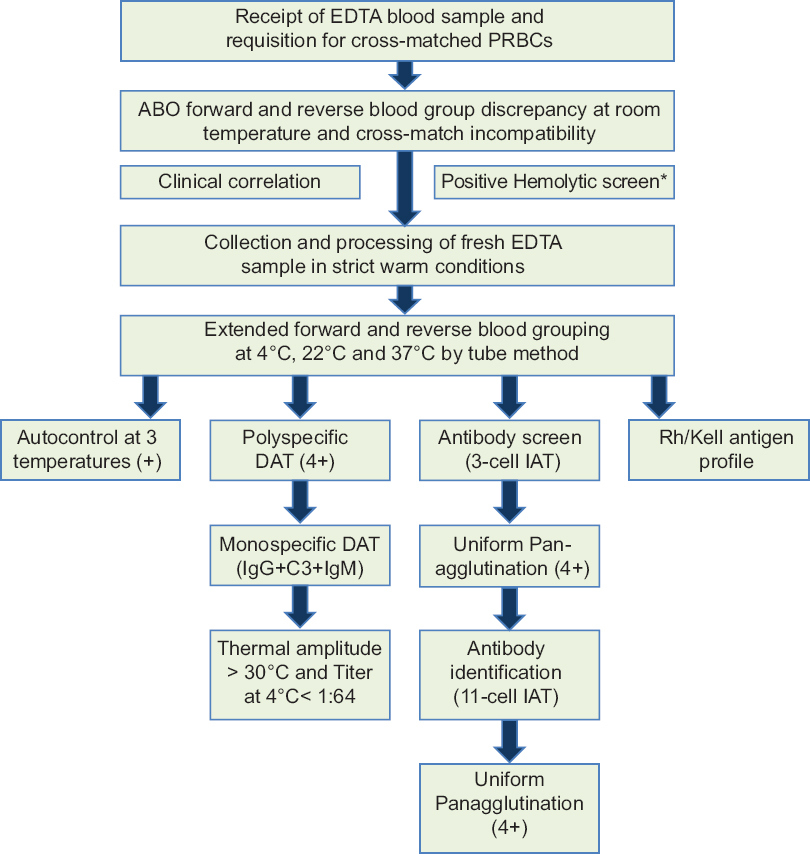
- Flowchart for immunohematological workup of both cases of mixed autoimmune hemolytic anemia. *Hemolytic screen (complete hemogram, peripheral smear, corrected reticulocyte count, serum lactate dehydrogenase, serum indirect bilirubin, and/or urine hemosiderin)
Discussion
Autoimmune hemolytic anemia (AIHA) was first described by Issit in 1985.[7] Warm AIHA more commonly affects children than cold AIHA. Warm autoantibodies are IgG type, acting at > 37°C, do not require complement activation and don't cause in vitro RBC agglutination. In contrast, cold autoantibodies are IgM type, acting at <37°C, require complement activation and cause in vitro RBC agglutination.[4] Cold autoantibodies commonly show specificity against the Ii blood group system, approximately 90% against I antigen.[8] Mixed AIHA is usually diagnosed when monospecific DAT is positive for warm IgG autoantibody and C3 and cold agglutinins with thermal amplitude >30°C with absence of characteristic features of cold hemagglutinin disease (CHAD).[345]
A positive DAT is the hallmark of immune hemolytic anemia, in approximately 95% cases. Rest 5% cases either have low-affinity antibody or too few antibody molecules coated on RBCs.[39] Among DAT positive cases, 20%–66% have only IgG, 24%–63% have both IgG and C3 while 7%–14% have only C3 coated on RBCs surface.[9] The IgM autoantibodies dissociate from RBCs subsequent to C3 binding and therefore are usually not detected in vitro.[10] Das et al. studied 43 cases of AIHA and found only 2 cases of mixed AIHA with IgG + IgM and 5 cases with IgG + IgM + IgA + C3.[11] RBCs coated with both IgG and complement undergo exaggerated extravascular hemolysis.[12] Such cases should also be screened for cold antibodies by mixing saline suspended RBCs in patient's serum at room temperature for 60 min (Direct Agglutination Test).[3]
Before diagnosing mixed AIHA, warm AIHA with clinically insignificant cold agglutinins and CHAD must be ruled out as their treatment is different. The cold agglutinins are usually active at <25°C with titer usually <1:64 at 4°C. While in CHAD, IgM antibody has the thermal amplitude ≥30°C and titer is usually >1:500 at 4°C, in appropriate clinical setting.[3] In both our cases, the cold autoantibody had high thermal amplitude and low titer.[3]
The role of monospecific DAT and indirect antiglobulin test (IAT) at 4°C and 37°C is paramount in the differentiation of warm versus cold antibodies. On IAT, uniform panagglutination is seen due to warm autoantibody at 37°C (in Coomb's phase) and cold autoantiboy at 4°C (in saline phase). Warm autoantibodies are usually directed against high incidence RBC surface antigens. However, such high incidence antigen-negative blood units are hardly available, so there is no point in further determining the antibody specificity.[2] Thermal amplitude and titer of the culprit autoantibody is directly proportional to the clinical severity and the speed of recovery, respectively.[7]
Approximately, one-third patients of AIHA have an underlying alloantibody (most commonly against Rh or Kell) particularly if there is a history of previous transfusion or pregnancy.[13] Hence, adsorption studies should be done to unmask alloantibody and determine its specificity. Although case 2 had history of transfusion, absence of graded reaction on IAT as well as absent mixed-field agglutination on Rh/Kell antigen profile contradicted the presence of alloantibody. Furthermore, adsorption studies are time consuming and not feasible in emergency situation.
A detailed work-up with close long-term follow-up are essential to rule out causes of secondary AIHA e.g. infections, lymphoproliferative malignancies or autoimmune diseases.[14]
Corticosteroid therapy and avoiding exposure to cold are the mainstay of treatment of mixed AIHA. Underlying disease should also be treated in secondary AIHA.[14] Transfusions are of limited benefit and must be given after careful assessment of risk–benefit ratio. Often the least incompatible, Rh/Kell-matched PRBCs are transfused that are rapidly destroyed by autoantibodies.[15] Since most RBCs express I antigen, cold autoantibody-mediated hemolysis might also be exaggerated. There is a risk of alloimmunization as well.[16] Furthermore, complement in the donor plasma might accelerate hemolysis.[14] Sometimes, transfusion might suppress compensatory erythropoiesis too, halting the recovery. Despite these risks, transfusion should not be denied in life-threatening conditions. Hence, at least DAT, antibody screen, and autocontrol should be performed in all AIHA cases to ensure safest possible transfusion.[17] In case, the autoantibody shows some specificity, the PRBCs negative for the corresponding antigen should be transfused. Iron and folic acid should be supplemented to compensate for hemolysis.[14] Usually, mixed AIHA has a chronic course with intermittent exacerbations. Rituximab and splenectomy might be beneficial in refractory cases.[418]
Conclusion
We are reporting these cases due to the extreme rarity of mixed AIHA in children. Furthermore, it may present as blood group discrepancy or cross-match incompatibility that poses a challenge to the immunohematologist. Often, warm AIHA with insignificant cold agglutinins is misdiagnosed as mixed AIHA; thus, determining thermal amplitude and titer is essential. The patients must be transfused judiciously with least incompatible, ABO and Rh/Kell phenotype-matched, prewarmed PRBCs given very slowly under steroid cover and strict clinical supervision.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The haemolytic anaemias. In: Firkin FC, Chesterman CN, Penington DG, Rush BM, eds. De Gruchy's Clinical Haematology in Medical Practice (5th ed). Edinburgh: Blackwell Science Ltd.; 1989. p. :172-215.
- [Google Scholar]
- British Society for Haematology. The diagnosis and management of primary autoimmune haemolytic anaemia. Br J Haematol. 2017;176:395-411.
- [Google Scholar]
- Hemolytic anemias resulting from extracellular factors. In: Behrman RE, Kliegman RM, Jenson HB, eds. Nelson Textbook of Pediatrics (18th ed). Philadelphia: Saunders; 2007. p. :2042-4.
- [Google Scholar]
- Autoimmune hemolytic anemia: Mixed type-a case report. Indian J Hematol Blood Transfus. 2011;27:107-10.
- [Google Scholar]
- Mixed- type autoimmune haemolytic anemia: Differential diagnosis and a critical review of reported cases. Transfusion. 2008;48:2229-34.
- [Google Scholar]
- Serological diagnosis and characterization of causative antibody. In: Chaplin H Jr, ed. Methods in Hematology – Immune Hemolytic Anemia. USA: Churchill Livingston; 1985.
- [Google Scholar]
- I blood group system and its relation to other blood group systems. J Med Lab Technol. 1967;24:90-7.
- [Google Scholar]
- The variability of hemolysis in the cold agglutinin syndrome. Blood. 1980;56:409-16.
- [Google Scholar]
- Clinical and serological characterization of autoimmune hemolytic anemia in a tertiary care hospital in North India. Ann Hematol. 2009;88:727-32.
- [Google Scholar]
- Autoimmune hemolytic anemia and paroxysmal nocturnal hemoglobinuria. In: Simon TL, Dzik WH, Synder EL, Stowell CP, Strauss RG, eds. Rossi's Principles of Transfusion Medicine (3rd ed). Philadelphia, USA: Lippincott Williams and Wilkins Publication; 2002.
- [Google Scholar]
- Review: Evaluation of patients with immune hemolysis. Immunohematology. 2004;20:167-76.
- [Google Scholar]
- Autoimmune hemolytic anemia: From lab to bedside. Asian J Transfus Sci. 2014;8:5-12.
- [Google Scholar]
- Blood transfusion in autoimmune haemolytic anemia: A practical problem. Indian Pediatr. 1993;30:264-6.
- [Google Scholar]
- The differentiation of delayed serologic and delayed hemolytic transfusion reactions: Incidence, long-term serologic findings, and clinical significance. Transfusion. 1990;30:688-93.
- [Google Scholar]
- Incompatible blood transfusion: Challenging yet lifesaving in the management of acute severe autoimmune hemolytic anemia. Asian J Transfus Sci. 2014;8:105-8.
- [Google Scholar]
- Mixed warm and cold autoimmune hemolytic anemia: Complete recovery after 2 courses of rituximab treatment. Blood. 2002;99:3478-9.
- [Google Scholar]





