Translate this page into:
Serum mannan and galactomannan level in culture-negative sepsis patients having suspected invasive fungal infections during COVID-19 pandemic: A clinico-microbiological study
*Corresponding author: Nilesh Kumar, Department of General Medicine, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India. nilesh19arreno@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Maurya VK, Gupta MK, Tilak R, Kumar K, Singh JK, Kumar P, et al. Serum mannan and galactomannan level in culture-negative sepsis patients having suspected invasive fungal infections during COVID-19 pandemic: A clinico-microbiological study. J Lab Physicians. 2025;17:113-8. doi: 10.25259/JLP_60_2024
Abstract
Objectives
Management of culture-negative sepsis in intensive care unit (ICU) admitted patients is difficult as these events are infectious and non-infectious in etiology. Thus, in the era of modern medicine, serological tests might help clinicians for better management of culture-negative sepsis events in ICU-admitted patients.
Materials and Methods
We prospectively enrolled 100 patients having culture-negative sepsis, admitted to the ICU during the COVID-19 pandemic. Baseline serum mannan and galactomannan (GM) levels by enzyme-linked immunosorbent assay-based method were determined in each patient. Concomitantly, C-reactive protein (CRP) and procalcitonin (PCT) values were also determined and the results were compared with other clinicoradiological evidence.
Statistical analysis
We determined the correlation between serum mannan and PCT, serum GM, and PCT by Spearman correlation coefficient.
Results
Baseline serum GM, mannan, PCT, and CRP were determined in 100 patients having culture-negative sepsis. A significantly higher mannan (125 pg/mL) was determined in 16 patients, whereas GM antigen (GM index [GMI] >0.5) was positive in 47 enrolled patients. 45 enrolled patients with higher GMI (>0.5) had respiratory symptoms. Out of 16 patients having higher mannan levels, only 3 had higher GMI. We also observed a significant negative correlation between serum mannan and PCT; a negative correlation between GMI and PCT; and a positive correlation between acute physiology and chronic health evaluation II score and PCT.
Conclusions
Composite tests alike serum mannan, GM, PCT, and CRP might be helpful in the management of culture-negative sepsis events in ICU-admitted patients.
Keywords
C-reactive protein
Medicine intensive care unit
Procalcitonin
Serum galactomannan
Serum mannan
INTRODUCTION
Sepsis is a life-threatening event in intensive care unit (ICU) admitted patients, where each hour delay in management increases the mortality.[1,2] Sepsis is infectious or non-infectious where rapid detrition of the body organ function occurs despite the administration of antimicrobial agents and other supportive measures.[1] Blood culture is the ideal investigation to determine the infective etiology of sepsis with its antibiogram.[3] However, low blood culture positivity is a major hindrance in evidence-based medicine as a larger portion of patients (~50%) fails to demonstrate a culture-proven source of infection (culture-negative sepsis).[4]
Culture negative sepsis is caused by non-cultivable or slow-growing bacteria, fungi, or viruses.[5] Moreover, the administration of broad-spectrum antibacterial and antifungals to ICU-admitted patients further reduces the blood culture positivity. Therefore, it is difficult to address the causative pathogens in patients suffering from sepsis. Serological markers at C-reactive protein (CRP) and procalcitonin (PCT) are widely used as presumptive markers for bacterial infections whereas serum mannan and galactomannan (GM) are used for presumptive diagnosis of fungal infections.[6] These serological tests had varied sensitivity and specificity as serum GM and β-D Glucan have high negative predictive values whereas CRP and PCT had higher positive predictive values for bacterial infections.[6,7]
During the COVID-19 pandemic, a rapid upsurge of bacterial and fungal infections was observed among ICU-admitted patients which were attributed to predisposing risk factors, use of steroids, and prolonged hospital stay.[8,9] In suspected invasive fungal infections (IFIs), blood culture is rarely positive, thus, composite serological testing is required in patients suffering from IFIs. Thus, a prospective study was done to determine the baseline serum CRP, PCT, mannan, and GM levels in patients suffering culture-negative sepsis.
MATERIALS AND METHODS
This present study was approved by the Institutional Ethical Committee Dean/2019/EC/1699 dated November 18th, 2019. We obtained the detailed demographic and clinical details from the ICU-admitted patients from January 2020 to December 2020. We had baseline investigations, complete blood count (CBC), liver function test (LFT), renal function test (RFT), arterial blood gas (ABG) analysis, acute physiology and chronic health evaluation (APACHE II), and sequential organ failure assessment (SOFA) scores at the time of ICU admission with their management in perspective of blood culture, serum CRP, lactate, PCT, mannan, and GM. Blood culture was done in the Automatic BacTalert continuous monitoring system. Serum GM was determined by sandwich enzyme-linked immunosorbent assay (ELSA)-based Platelia™ Aspergillus Ag kit, whereas Serum mannan was determined by ELSA-based Platelia™ Candida Ag as per the manufacturer’s instructions. For estimation of the GM antigen, GM index (GMI) (patient’s serum optical density (OD)/cut off OD) was determined, where GMI of ≥0.5 was considered positive. For estimation of the serum mannan, a value of 125 pg/mL (kit instruction) was considered positive. We grouped these enrolled patients into two: Survived and non-survived groups and analyzed the data.
We checked the normality of the distributions of the obtained data in the study. We performed all statistical analyses using MINITAB 16 (Minitab Inc., State College, Pennsylvania, USA), where P < 0.05 was considered statistically significant. We presented the data as mean ± standard deviation (SD) for parametric continuous variables and frequency with their respective percentages for categorical variables, where the Chi-square test was used. Correlation between the CRP, PCT, mannan, and GM was calculated using Spearman rho correlation as the data are non-normally distributed.
RESULTS
Of enrolled 100 ICU-admitted patients having culture-negative sepsis, 51 were male. Most of the enrolled patients belonged to 41–60 years of age group. These enrolled patients had varied clinical presentations: fever (n = 96), cough (n = 88), difficulty in breathing (n = 86), and altered sensorium in 40 patients. On examination, 84% of the enrolled patients had involvement of the respiratory system followed by the central nervous system. These enrolled patients were divided into two groups: Survived (n = 48) and non-survived (n = 52). A biostatistical significance difference for SOFA and APACHE II scores was observed among the non-survival patients as these patients had high scores. Among non-survived patients, 86.5% of patients were on mechanical ventilation, whereas 69.02% and 57.7% of patients had immunocompromised states and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, respectively. The predisposing risk factors associated with mortality have been shown in Table 1, where a biostatistical significance was observed for SARS-CoV-2 infection, immunocompromised states, patients on vasopressor support, and mechanical ventilation. While, among 52 non-survivals, 69.2% and 57.7% of patients had predisposing risk factors of immunosuppression and SARS-CoV-2 infection, respectively. Other risk factors such as diabetes mellitus, neutropenia, chronic kidney disease, chronic liver diseases, malignancies, and hypertension were not significantly associated with mortality among ICU-admitted patients having culture-negative sepsis.
| Risk factors | Outcome | P-value | |||
|---|---|---|---|---|---|
| Survived | Not survived | ||||
| No. | %. | No. | %. | ||
| Diabetes mellitus | 18 | 37.5 | 25 | 48.1 | 0.286 |
| Neutropenia | 8 | 16.7 | 11 | 21.2 | 0.568 |
| SARS-CoV-2 infection | 9 | 18.8 | 30 | 57.7 | <0.001 |
| Seropositive for HIV antibodies | 2 | 4.2 | 1 | 1.9 | 0.511 |
| Hypertension | 17 | 35.4 | 17 | 32.7 | 0.774 |
| COPD | 3 | 6.3 | 6 | 11.5 | 0.356 |
| Patients on ventilator support | 16 | 33.3 | 45 | 86.5 | <0.001 |
| Patients on immunosuppressant drugs | 15 | 31.3 | 36 | 69.2 | <0.001 |
| Vasopressor Support | 8 | 16.7 | 23 | 44.2 | 0.003 |
| Other (CKD, CLD, Malignancy) | 7 | 14.6 | 11 | 21.2 | 0.393 |
COPD: Chronic obstructive pulmonary disorders, CKD: Chronic kidney disease, CLD: Chronic liver disease, SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2, HIV: Human immunodeficiency virus
In all 100 enrolled patients, serum GM was determined by Biorad Platellia Aspergillus antigen Kit using a Sandwich enzyme-linked immunosorbent assay, where GMI of ≥0.5 was considered positive. A total of 47 patients had a higher GMI which had predisposing risk factors of immunocompromised state and SARS-CoV-2 infection [Table 2]. However, serum mannan was positive in 42% (16/38) of the enrolled patients. We also assessed the serum mannan levels in the patients having significant GMI (>0.5), where a biostatistical difference was observed between the serum mannan and significant GMI [Table 3].
| Survived Mean±SD n=48 | Non-survived mean±SD n=52 | P-value | |
|---|---|---|---|
| Age | 50.77±14.498 | 56.94±11.593 | 0.020 |
| Duration of stay | 12.521±7.6963 | 14.981±7.3070 | 0.104 |
| SGOT (U/L) | 104.188±183.2429 | 270.192±454.4932 | 0.020 |
| SGPT (U/L) | 99.938±209.4616 | 229.962±395.0479 | 0.045 |
| INR | 1.331±0.2256 | 1.544±0.6225 | 0.028 |
| Lactate (mMol/L) | 2.838±2.6797 | 3.966±3.5570 | 0.078 |
| PCT (ng/mL) | 2.9531±3.91138 | 7.2569±12.49912 | 0.025 |
| CRP (mg/L) | 71.979±35.4206 | 91.869±50.1117 | 0.025 |
| Mannan value (pg/mL) | 102.583±43.0020 | 84.692±42.2040 | 0.235 |
| APACHE II score | 9.667±5.1420 | 17.692±5.7105 | <0.001 |
| SOFA score | 5.625±2.7493 | 10.173±3.2883 | <0.001 |
SGOT: Serum glutamic-oxaloacetic transaminase, SGPT: Serum glutamic-pyruvic transaminase, INR: International normalized ratio, PCT: Procalcitonin, CRP: C-reactive protein, APACHE: Acute physiology and chronic health evaluation, SOFA: Sequential organ failure assessment, SD: Standard deviation
| GM index | Outcome | |||
|---|---|---|---|---|
| Survived | Not survived | |||
| No. | %. | No. | %. | |
| <0.5 | 26 | 49.1 | 27 | 50.9 |
| 0.5–0.99 | 11 | 100 | 0 | 0.0 |
| 1–1.49 | 2 | 28.6 | 5 | 71.4 |
| 1.5–2 | 6 | 35.3 | 11 | 64.7 |
| >2 | 3 | 27.3 | 9 | 72.7 |
| Total | 48 | 100 | 52 | 100 |
χ2=16.642, P=0.001, GM: Galactomannan
Concomitantly, serum PCT and serum CRP levels were determined in all enrolled patients. In comparison to the survivors, a biostatistical significant higher serum PCT and CRP was noted in non-survivors [Table 2]. Moreover, a negative correlation between mannan value and PCT (P < 0.018) was also observed [Figure 1]. Thus, the four serological tests were performed in ICU-admitted patients having culture-negative sepsis. We observed a statistically significant negative correlation between GMI and PCT [Figure 2]. We also observed a positive correlation between APACHE II score and PCT; APACHE II score and lactate; APACHE II score and CRP; SOFA score and lactate; SOFA score and CRP; and SOFA score and PCT. We observed that increasing GMI is significantly associated with mortality among ICU-admitted patients [Table 3].
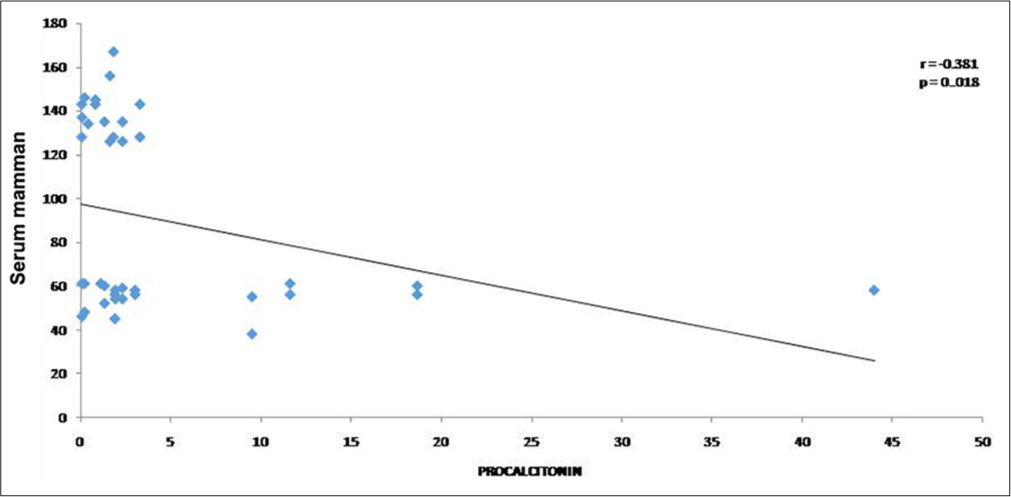
- Spearman correlation of serum mannan and procalcitonin.

- Spearman correlation of serum galactomannan index and procalcitonin.
DISCUSSION
Culture-negative sepsis is a frequently encountered phenomenon in ICU settings where the selection of the next path of management is difficult for physicians. This culture-negative sepsis may be attributed to different factors; administration of antimicrobial agents, non-culturable/slow-growing microbes as a causative agent, or in a condition of deep-seated abscess.[10,11] During the COVID-19 pandemic from April 2020–September 2021, an increased incidence of bacterial and fungal infections was reported from the ICU-admitted patients.[8,12] These disseminated/deep-seated infections are usually diagnosed by blood culture, which has a low positivity.
In this present study, we enrolled 100 patients with blood culture-negative sepsis, admitted in ICU settings in the COVID-19 era. Based on the final treatment outcome, these patients were divided into two groups and compared to the baseline investigations and serological tests. We observed a significantly higher mortality in the patients having SARS-CoV-2 infection, immunocompromised state, and patients on mechanical ventilation as predisposing risk factors. During the pandemic of SARS-CoV-2 infection, an increased incidence of respiratory fungal infection (aspergillosis/mucormycosis) has been reported from different parts of the world.[12] Moreover, bacterial and yeast systemic infections have been frequently observed during this pandemic.[11,12] This increased incidence of fungal infection in the COVID-19 pandemic may be attributed to the widespread use of steroids, interleukin-6 inhibitor (Tocilizumab), and a broad spectrum of antibiotics.[13] SARS-CoV-2 virus also causes endothelitis and mucositis, favoring the invasion of fungal pathogens.[13]
These bacterial/fungal infections are usually diagnosed by blood culture. Despite having a low positivity, blood culture is the cornerstone of the management. In the case of positive blood culture, the isolated causative microbes with their antibiogram guide the physicians for better management of the sepsis event. In contrast, negative blood cultures in ICU-admitted patients, having clinical signs and symptoms of sepsis, are common. In such conditions, it is very difficult to select the appropriate antimicrobials as sepsis is infectious or non-infectious; therefore, there is a need for an appropriate diagnosis. Slow growing/non-cultivable bacterial and fungal pathogens are the common cause of culture-negative sepsis creating a dilemma for treating physicians.
Serological markers may overcome the dilemma, faced by the physician. These serological markers, although not specific, help in the presumption of the microbial etiology. PCT and CRP are widely used for the presumption of bacterial infections, whereas serum mannan, GM, and β-D-Glucan are used for IFIs. However, the sensitivity and specificity of these tests vary. Serum PCT aids the early diagnosis of sepsis where it is also used to monitor the treatment response. The normal serum PCT level in healthy individuals is usually <0.1 μg/L. A degree of raised PCT (>0.5 μg/L) is usually associated with the severity of bacterial infections. This raised PCT depends on the microbial etiology of sepsis as a higher serum PCT level has been reported in Gram-negative sepsis, in comparison to Gram-positive.[14] For diagnosis of IFI, its sensitivity is poor. However, its low serum level in candidemia cases has been reported.[15]
Mannan, a cell wall component of the Candida spp., is quantified in the serum of the patients having invasive candidiasis. This antigen is readily degraded and eliminated from the bloodstream, therefore concomitantly antimannan antibodies are to be determined.[16] In the present study, we only determined serum mannan level with PCT and CRP serological markers. We observed an inverse correlation between the serum mannan and serum PCT as (r = −0.02, P = 0.018). We also assessed the correlation between the serum CRP and serum mannan and observed an inverse correlation between these two variables. Thus, a combination of serum mannan and CRP/PCT may help in differentiating IFI in ICU-admitted patients.
Serum GM is the cell wall component of the Aspergillus spp., which is released in body fluids during its hyphal growth.[17] Serum GM is used frequently in the diagnosis of invasive aspergillosis, despite being less sensitive in comparison to bronchoalveolar lavage (BAL) GM.[18] Moreover, the level of serum GM is also influenced by the administration of the beta-lactam group of antibiotics, cereal and cereal food consumption, and the prophylactic use of antifungal agents.[17] Thus, serum GM has a high negative predictive value where negative results exclude the possibility of invasive aspergillosis. In the present study, most of the admitted patients had a higher GMI (≥0.5). Moreover, we also determined the serum PCT and CRP and observed an inverse correlation between the serum GM and PCT (r = −0.263, P = 0.008) or CRP. While comparing the two fungal seromarkers (serum mannan and GM), we observed that serum mannan was raised in the patients, in whom the serum GM was negative or had a cut-off of 1. Thus, a combination of these four serological tests can help in the management of culture-negative patients, where raised PCT and CRP may predict bacterial infection. In contrast, raised serum mannan or GM with low PCT or CRP may predict the IFI.
In this present study, we observed higher mortality among the ICUs admitted patients having predisposing risk factors such as old age and co-morbid conditions. In our study, the patients who had high GMI succumbed to death despite the administration of voriconazole in appropriate doses as voriconazole is considered a first-line drug for invasive aspergillosis.[19] However, its trough level is altered by the concomitant administration of enzyme inducers and inhibitors.[20] Thus, correction of underlying predisposing risk factors and monitoring of voriconazole trough level may result in favorable outcomes; however, varied MIC against Aspergillus fumigatus and Aspergillus flavus species have been reported in the literature.[20] In the present study, we observed a slightly reduced mortality in patients who were positive for serum mannan antigen. In such cases, amphotericin B is the drug of choice.[21]
Mortality in ICU-admitted patients can be reduced by the selection of appropriate antimicrobial agents. These patients usually had blood culture-negative sepsis, where concomitant determination of different serological markers such as CRP, PCT, serum mannan, and GM helps in the management of sepsis. Moreover, the molecular-based diagnosis of the IFIs can be done. Molecular tests are laborious, costly and may give false positive results in conditions of fungal contamination.[22] Henceforth, in this study, we have quantified four serological markers in the serum of the enrolled patients, we did not quantify the other markers as β-D-Glucan. We did not categorize these enrolled patients into the proven, probable IFIs. The strength of the study is that the use of a combination of different seromarkers can identify the microbial etiology of culture-negative sepsis. However, the exact etiology is a need of the hour.
CONCLUSIONS
Higher serum PCT and CRP levels usually indicate the bacterial etiology of culture-negative sepsis. However, raised serum mannan and GM with normal-low PCT and CRP usually indicate fungal infections. Thus, concomitant use of these four serological markers may help the physician select antimicrobial agents in ICU-admitted patients having culture-negative sepsis.
Author contribution
NK, MKG, KK: Conceptualization; VKM, PK, MKG, RT, NK, KK: Methodology; VKM, PK, MKG, RT, NK, KK: Formal analysis and investigation; VKM, PK, MKG, NK: Writing - original draft preparation; VKM, PK, MKG, RT, NK, KK: Writing - review and editing; NK, KK, RT: Supervision.
Ethical approval
The research/study was approved by the Institutional Ethical Committee at the Institute of Medical Sciences, number 1699, dated 18th November 2019.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Critical care in the emergency department: severe sepsis and septic shock. Emerg Med J. 2006;23:713-7.
- [CrossRef] [Google Scholar]
- Impact of delayed antimicrobial therapy in septic ITU patients. Crit Care. 2010;14:1-2.
- [CrossRef] [Google Scholar]
- Monitoring infection: from blood culture to polymerase chain reaction (PCR) Best Pract Res Clin Anaesthesiol. 2013;27:279-88.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of positive blood cultures in critically ill patients: A retrospective evaluation. Croat Med J. 2012;53:30-9.
- [CrossRef] [PubMed] [Google Scholar]
- Biomarkers of fungal infection: Expert opinion on the current situation. Rev Esp Quimioter. 2020;33:1-10.
- [CrossRef] [PubMed] [Google Scholar]
- Bacterial and fungal infections in COVID-19 patients: A matter of concern. Infect Control Hosp Epidemiol. 2020;41:1124-5.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19-associated mucormycosis: A clinico-epidemiological study. J Diabetes Complications. 2022;36:108284.
- [CrossRef] [PubMed] [Google Scholar]
- Unrevealing culture-negative severe sepsis. Crit Care. 2013;17:1001.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic challenges in sepsis. Curr Infect Dis Rep. 2021;23:22.
- [CrossRef] [PubMed] [Google Scholar]
- Secondary infections in hospitalized COVID-19 Patients: Indian experience. Infect Drug Resist. 2021;14:1893-903.
- [CrossRef] [PubMed] [Google Scholar]
- Breakthrough invasive mold infections in the hematology patient: Current concepts and future directions. Clin Infect Dis. 2018;67:1621-30.
- [CrossRef] [PubMed] [Google Scholar]
- Procalcitonin levels in gram-positive, gram-negative, and fungal bloodstream infections. Dis Markers. 2015;2015:701480.
- [CrossRef] [PubMed] [Google Scholar]
- Procalcitonin levels in candidemia versus bacteremia: A systematic review. Crit Care. 2019;23:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of (1→3)-β-d-glucan, mannan/anti-mannan antibodies, and Cand-Tec Candida antigen as serum biomarkers for candidemia. J Clin Microbiol. 2013;51:1158-64.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of serum and bronchoalveolar lavage fluid galactomannan in diagnosis of chronic pulmonary aspergillosis. J Clin Microbiol. 2019;57:e01821-18.
- [CrossRef] [PubMed] [Google Scholar]
- Necessity to identify the causative agent for appropriate treatment in fungal corneal ulcer: An in vitro study. J Mycol Med. 2018;28:201-5.
- [CrossRef] [PubMed] [Google Scholar]
- Observational study of associations between voriconazole therapeutic drug monitoring, toxicity, and outcome in liver transplant patients. Antimicrob Agents Chemother. 2017;61:e01211-17.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of nested polymerase chain reaction for fungus in detecting clinically suspected patients of invasive fungal infections and its clinical correlation and comparison with fungal culture. J Family Med Prim Care. 2020;9:4992-7.
- [CrossRef] [PubMed] [Google Scholar]






