Translate this page into:
Study of the Stability of Various Biochemical Analytes in Samples Stored at Different Predefined Storage Conditions at an Accredited Laboratory of India
Address for correspondence: Dr. Kamal Kachhawa, E-mail: drkamalkachhawa@yahoo.ca
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Storage of serum and other blood products is often necessary in laboratories because of technical issues or to preserve samples for subsequent research purposes. The aim of this study was to determine whether the stability of biochemical analytes is affected by storage conditions.
Materials and Methods:
A total of 17 biochemical analytes in the sera of ten patients were examined following storage. Subsequent to determining the baseline measurements, the serum of each patient was aliquoted and stored at −20°C for 7, 15, and 30 days and then analyzed for stability. The results were compared with the initial analysis measurements obtained from fresh samples. Mean changes compared to baseline (T0) concentrations were evaluated both statistically and clinically.
Results:
Our results show that sodium, potassium, urea, creatinine, uric acid, total calcium, phosphorus, direct bilirubin, total bilirubin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, total protein, albumin, cholesterol, and triglyceride levels were stable under all conditions. Serum amylase was the only analyte demonstrating instability following prolonged storage; amylase levels changed significantly (both statistically and clinically) at 7, 15, and 30 days (P < 0.05).
Conclusion:
Most common biochemical analytes, except for amylase, showed adequate stability in serum following 30 days of storage at −20C. Serum amylase analysis should be conducted on the same day that the sample is received in the laboratory.
Keywords
Amylase
biochemical analytes
serum
stability
temperature
INTRODUCTION
Acommon problem in clinical laboratories is maintaining the stability of serum analytes during sample storage. Samples are usually stored in the door (4–8°C) of a refrigerator for short durations or in a deep freezer (−20°C) for longer time periods. Thus, the temperature at which the samples are stored constitutes an important preanalytical variable that may affect analysis results in the clinical biochemistry laboratory setting.
Tertiary care hospital laboratories received over a thousand samples a day. These laboratories face many challenges including equipment breakdown and the lack of reagents, which can prevent same-day processing of samples. In such cases, the only option is to preserve the samples in a deep freezer (−20°C). In addition, samples are sometimes stored for an extended duration until subjected to routine batch analysis for research purposes. Previous studies have provided information regarding the stability of analytes in serum using a number of methods[12] that have since become obsolete. Although many blood analytes have been shown to deteriorate within hours in unseparated samples kept at ambient temperature, the few studies examining unseparated samples stored at low temperatures involved prolonged contact between serum and cells, which could have caused erroneous test results.[3] Moreover, most of these older studies were conducted with animal samples.[4]
The stability of 72 analytes following prolonged serum-cell contact has been previously described.[12] The effects of prolonged storage on the stability of 31 analytes in plasma and serum separated from cells with a gel barrier have also been reported.[56] A number of studies have described approximately thirty analytes in serum immediately separated from cells.[78] In addition, several studies have examined the effect of storage conditions on the stability of various serum components.[91011]
However, limited information is available regarding the stability of commonly used clinical biochemical analytes in human serum including the effect of storage temperatures as low as −20°C on blood-separated serum. Therefore, the present study examined the stability of 17 routine chemistry analytes in immediately cell-separated serum following storage at a designated temperature (−20°C) for different periods (0, 7, 15, and 30 days) using the previously described standard guidelines for blood sample handling and separation.[12]
MATERIALS AND METHODS
Study design
This hospital-based study included ten random samples from outpatients being treated at the hospital clinics. The samples collected from each patient were for physician-ordered laboratory testing; no additional blood was taken from the subjects. The Institutional Ethical Committee approved the study, and informed consent was obtained from all the participants. All procedures were conducted in accordance with the guidelines of the Helsinki declaration on human experimentation.
Sample collection and analysis
Fasting venous blood (total of 6 mL blood) was collected in the morning using a Vacuette® standard tube holder and Vacuette® 22GA × 1” (0.7 mm × 25 mm) multisample needle (Becton, Dickinson and Company, USA). The blood specimens were drawn into 7.5 mL plastic Vacuette® serum tubes (BD Vacutainer® serum; BD, Franklin Lakes NJ, USA).
The sample tubes were left in an upright position for 30 min at room temperature followed by centrifugation at 3500 rpm for 10 min. Serum samples were examined for hemolysis and lipemia to prevent possible interference. The serum samples of each subject were pooled into a plain tube and then aliquoted into 1.5 mL Eppendorf tubes (Eppendorf, Milano, Italy); four aliquots per patient samples were kept (three for storage at −20°C) and the remaining serum was used for the baseline measurement (T1d). All samples were kept frozen until experimental analysis. The serum aliquots were stored frozen at −20°C for either 7 (T7d), 15 (T15d), or 30 (T30d) days and then analyzed separately for stability.
The following analytes were examined: [Table 1]
-
Metabolites: Na+, K+, urea, creatinine, uric acid, total calcium, phosphorus, direct bilirubin, and total bilirubin
-
Proteins: Total protein and albumin
-
Lipids: Total cholesterol and triglycerides
-
Enzymes: Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and amylase.
All measurements were performed at the hospital laboratory services using an Olympus AU 400 auto analyzer, except for serum electrolyte levels, which were obtained with a Roche AVL electrolyte analyzer.
Statistical analysis
To determine time-dependent changes in cell-separated serum analytes, the mean value from all ten subject samples was calculated for each analyte at each time point. Statistically significant changes were determined for each analyte by repeated-measures ANOVA. Clinically significant changes were determined using the significant change limit (SCL)[13] approach, defined as: SCL = initial value ± 3.0 usual standard deviation (USD). This is based on the assumption that the USD is representative of the inherent day-to-day variability of the method. In our study, the calculated mean for each analyte at T1d represented the initial value. The USD was obtained by averaging the standard deviation of the quality control data of the previous 2 months for each analyte.[14] The quality-control reference serum with the target mean most closely matching the T1d mean for each analyte was used to determine the USD. For simplicity, the SCL was computed for each analyte by establishing the range (±3.0 USD) from the subject mean at T1d. Statistical analyses were performed with SPSS Version 17 (SPSS Inc., 233, Chicago, IL).
RESULTS
The analysis results for 17 biochemical parameters measured in serum samples under different storage conditions are shown in Table 2.
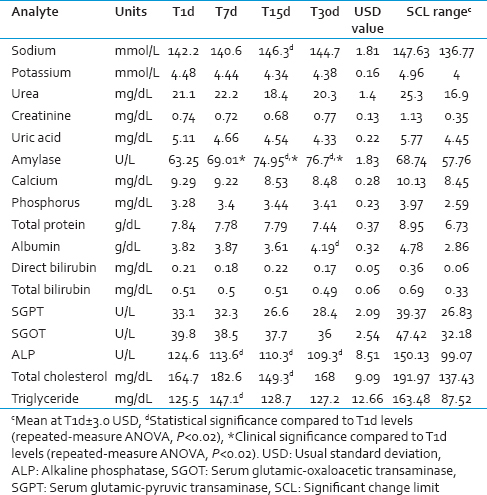
No significant statistical or clinical differences were found among most of the metabolites (Na+, K+, urea, creatinine, uric acid, total calcium, phosphorus, direct bilirubin, and total bilirubin) under the different storage conditions.
The changes in Na+ on T15d were statistically but not clinically significant. Albumin also demonstrated statistically significant variation on T30d compared with fresh sera on T1d; however, this variation was not clinically significant.
Aside from amylase, none of the enzymatic parameters exhibited clinically significant reduction inactivity. Sera stored for different periods at −20°C showed a statistically significant decrease in ALP; however, this trend was not clinically significant. The effect of storage at lower temperatures on serum amylase was statistically as well as clinically significant.
The changes in serum cholesterol and serum triglyceride were statistically significant on T15d and T7d, respectively, compared to T1d; however, they were not clinically significant. This may be attributed to systematic errors occurring on the respective days.
DISCUSSION
Metabolites
The results of our study indicate that nearly all of the examined metabolites were stable even after 30 days of storage at −20°C. In agreement with the findings of Zhang et al.,[12] no clinically significant differences were found for sodium levels following different storage durations compared to fresh samples; however, our serum Na+ findings are not consistent with the findings of studies investigating serum with prolonged contact with cells at room temperature.[212] Serum potassium was found to be stable up to T30d when the serum samples were separated from cells and stored in aliquots, whereas previous studies have demonstrated an increase in K+ after 24 h due to serum-cell contact at room temperature.[25] The increase in K+ after 24 h is most likely caused by malfunction of the Na+/K+ ATPase pump, resulting in diffusion of K+ from the erythrocytes driven by the intracellular–extracellular concentration gradient.[15]
Moreover, our results showed a clinically insignificant increase in Na+ level at T15d and in urea, calcium, total bilirubin, and direct bilirubin at T30d. However, no significant statistical or clinical differences were observed between the levels of the metabolites (Na+, K+, urea, creatinine, uric acid, total calcium, phosphorus, direct bilirubin, and total bilirubin) in fresh samples and in samples stored at −20°C for 7, 15, and 30 days. These results are in agreement with those of a previous study[16] reporting that serum calcium, total bilirubin, and direct bilirubin showed clinically equivalent levels, but that serum urea levels exhibited an appreciable increase in BUN values over time;[16] however, BUN instability, indicated by a substantial decrease (15.6% on average) in levels, has been reported for samples stored at −20°C.[17]
Consistent with a previous study,[17] we did not detect any statistically or clinically significant change in creatinine levels. However, according to Boyanton and Blick, the increase in serum creatinine levels after 24 h is due to serum-cell contact at room temperature.[3] In contrast to previous studies[1416] demonstrating that serum uric acid concentrations were unstable after 48 h of storage at −4°C, our results showed a serial decrease in uric acid concentration over time; however, these changes were neither statistically nor clinically significant. Furthermore, our results demonstrating a slight increase in phosphorus concentration (not statistically or clinically significant) disagree with previous studies showing that serum phosphorus concentrations increased after 24 h.[35]
Protein
No differences in serum protein (total protein and albumin) were detected at any of the three time points compared to fresh samples. Interestingly, Zhang et al.[12] noted similar observations for albumin and total protein in serum specimens. Moreover, our results are similar to a previous study[16] reporting clinically equivalent levels of total protein and albumin.
Lipid
Total cholesterol and triglyceride concentrations were stable up to T30d, in agreement with the findings of Cuhadar et al. and Paltiel et al.[1618]
Enzymes
A decrease in the concentration of serum ALT, AST, and ALP was observed; however, these changes were not statistically or clinically significant. Our findings regarding serum AST are consistent with those of Cuhadar et al.[16] In addition, according to Paltiel et al.,[18] AST activity remains stable for 10–15 freeze-thaw cycles following storage at −80°C. Significant statistical or clinical differences were observed between serum amylase levels in fresh samples and samples stored at −20°C for 7, 15, and 30 days; serum amylase levels decreased with prolonged storage. To the best of our knowledge, this is the first report regarding the effects of storage at low temperatures on serum amylase levels.
CONCLUSION
All common clinical chemistry analytes examined, aside from serum amylase, showed adequate stability following up to 30 days storage at −20°C. These results indicate that deep freezing at −20°C could serve a useful tool for additional analyses at later time points as well as for research purposes, which require that samples be stored for longer periods until batch analysis can be conducted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are indebted to Prof. Dr. Dinesh Puri, Professor and Head of the Department of Biochemistry, UCMS. The authors are also grateful to Dr. Archana Singh, Assistant Professor, Department of Biochemistry, AIIMS, New Delhi, and to the colleagues, participants, and laboratory technicians who took part in this study for their valuable support and cooperation.
REFERENCES
- Changes in serum chemical values as a result of prolonged contact with the clot. Am J Clin Pathol. 1976;66:598-604.
- [Google Scholar]
- Serum-constituents analyses: Effect of duration and temperature of storage of clotted blood. Clin Chem. 1981;27:35-8.
- [Google Scholar]
- Stability studies of twenty-four analytes in human plasma and serum. Clin Chem. 2002;48:2242-7.
- [Google Scholar]
- Effect of repeated freezing and thawing on 18 clinical chemistry analytes in rat serum. J Am Assoc Lab Anim Sci. 2012;51:475-8.
- [Google Scholar]
- Storage of serum or whole blood samples? Effects of time and temperature on 22 serum analytes. Eur J Clin Chem Clin Biochem. 1995;33:231-8.
- [Google Scholar]
- Storage of plasma in primary plasma separator tubes. Ann Clin Biochem. 1993;30(Pt 2):213-4.
- [Google Scholar]
- The stability of blood, plasma and serum constituents during simulated transport. Scand J Clin Lab Invest. 1981;41:35-40.
- [Google Scholar]
- Stability of serum and blood constituents during mail transport. Scand J Clin Lab Invest. 1981;41:425-30.
- [Google Scholar]
- The effect of freezing, thawing, and short- and long-term storage on serum thyrotropin, thyroid hormones, and thyroid autoantibodies: Implications for analyzing samples stored in serum banks. Clin Chem. 2007;53:1986-7.
- [Google Scholar]
- Effect of freeze-thaw cycles on serum measurements of AFP, CEA, CA125 and CA19-9. Scand J Clin Lab Invest. 2007;67:741-7.
- [Google Scholar]
- Effects of sample handling and storage on quantitative lipid analysis in human serum. Metabolomics. 2009;5:507-16.
- [Google Scholar]
- Effect of Serum clot contact time on clinical chemistry laboratory results. Clin Chem. 1998;44:1325-33.
- [Google Scholar]
- Quality control for the clinical chemistry laboratory. In: Kaplan LA, Pesce JA, eds. Clinical Chemistry: Theory, Analysis, and Correlation (3rd ed). Louis, MO: CV Mosby Company; 1996. p. :385-91.
- [Google Scholar]
- Stability studies of common biochemical analytes in serum separator tubes with or without gel barrier subjected to various storage conditions. Biochem Med (Zagreb). 2012;22:202-14.
- [Google Scholar]
- Physiology of red blood cells. In: Blood: A Textbook of Hematology (2nd ed). Boston, MA: Little Brown and Company; 1996. p. :157-77.
- [Google Scholar]
- The effect of storage time and freeze-thaw cycles on the stability of serum samples. Biochem Med (Zagreb). 2013;23:70-7.
- [Google Scholar]
- Long-term stability of biochemical markers in pediatric serum specimens stored at –80°C: A CALIPER substudy. Clin Biochem. 2012;45:816-26.
- [Google Scholar]
- Evaluation of freeze thaw cycles on stored plasma in the biobank of the Norwegian mother and child cohort study. Cell Preserv Technol. 2008;6:223-30.
- [Google Scholar]





