Translate this page into:
Traversing Their Path to the Peripheral Smear: The Journey of Traumatized Red Blood Cells
Address for correspondence: Chethana Mannem, MD, Department of Pathology, Kempegowda Institute of Medical Sciences, Bangalore 560004, Karnataka, India (e-mail: jaishnavsai@gmail.com).
-
Received: ,
Accepted: ,
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Thrombotic microangiopathy encompasses a wide range of conditions, of which thrombotic thrombocytopenic purpura being a medical emergency requires prompt intervention, with schistocytes being a reliable morphological indicator of microvascular injury. However, there are conditions other than thrombotic microangiopathic anemia where schistocytes can be seen in large numbers. These nonthrombotic microangiopathic conditions are broadly grouped under cytoskeletal abnormalities, mechanical damage, and thermal injuries. Automated methods in schistocyte evaluation have shown varied reproducibility requiring manual identification. International Council for Standardization in Hematology (ICSH) recommends standardized morphological criteria and quantitative assessment as a percentage after counting at least 1,000 red blood cells in optimal areas of smear to reduce interobserver variability.
Objectives
The aim of this study was to evaluate and quantitate schistocytes in thrombotic microangiopathic and nonthrombotic microangiopathic groups using ICSH guidelines and to evaluate interobserver reproducibility of manual schistocyte count.
Materials and Methods
Overall, 157 peripheral blood smears showing schistocytes were studied by two independent observers using ICSH recommendations on light microscopy. The hematological findings were correlated with clinical diagnosis and other relevant investigations.
Results
Schistocytes were observed in five cases of thrombotic microangiopathic anemia and 152 cases of nonthrombotic microangiopathic anemia. Schistocyte count in thrombotic microangiopathic anemia and nonthrombotic microangiopathic anemia groups with mean (±standard deviation) value was 2.28 ± 2.65% and 0.76 ± 0.67%, respectively (p < 0.001). The correlation coefficient between the two observers was 0.59 (confidence interval = 0.966–1.346) showing an excellent agreement on the reproducibility of schistocytes by application of ICSH guidelines.
Conclusion
Percentage of schistocytes more than 1% is a robust morphological indicator for diagnosis of thrombotic microangiopathic anemia in adults. Strict application of ICSH guidelines reduces interobserver bias.
Keywords
ICSH
schistocytes
thrombotic microangiopathy
thrombotic
thrombocytopenic
purpura
Introduction
Thrombotic microangiopathies (TMAs) are a group of disorders characterized by intravascular thrombi resulting in red cell fragmentation—schistocytes, which form as a consequence of mechanical damage when forced through a fibrin meshwork. However, schistocytes are absent or very rare in peripheral blood smear (PBS) of healthy individuals.[1,2] TMA includes thrombotic thrombocytopenic purpura (TTP), hemolytic uremic syndrome, disseminated intravascular coagulopathy, and hemolysis with elevated liver enzymes and low-platelet syndrome. Of these, TTP is a medical emergency requiring early diagnosis and prompt treatment with plasma exchange.[3] Diagnosis requires sophisticated and time-consuming investigations resulting in schistocyte identification in PBS as the morphological hallmark.[1–3] However, schistocytes are not specific to TMA and are seen in large numbers in non-TMA conditions, which can be broadly categorized under cytoskeletal abnormalities such as hemoglobinopathies, and mechanical damage as in prosthetic heart valves and in patients on dialysis and thermal injury.[2] Despite the occurrence of schistocytes in smears, laboratory surveys in identification of schistocytes remain difficult and subjective due to technical factors and under/over-interpretation with other poikilocytes. Lack of standardization may lead to inconsistency and misdiagnosis, thereby affecting treatment and clinical outcome. Thus, its utility in clinical settings and correct identification needs to be emphasized.[1,2,4]
Hence, International Council for Standardization in Hematology (ICSH) Schistocyte Working Group has recommended strict morphologic criteria and quantification of schistocytes.
Schistocytes are red cell fragments having sharp angles, straight borders/helmet cells, small crescents, and keratocytes. Schistocyte percentage above 1% after counting 1,000 red blood cells (RBCs) on peripheral smear is required for the diagnosis of TMA.[1,2] With automated analyzers being widely used, many present-day analyzers provide information on fragmented red cells. However, they have shown varied reproducibility, requiring manual identification.[2,4]
Objectives
To evaluate and quantitate schistocytes in TMA and non-TMA groups using ICSH guidelines.
To evaluate interobserver reproducibility of manual schistocyte count.
Materials and Methods
A hospital-based cross-sectional study was performed at a tertiary care center over a period of 6 months, from January 2021 to June 2021, after approval by Institutional Ethics Committee.
Following informed consent, the samples were received from patients at the central laboratory for basic hematologic work-up. Blood samples requiring examination of peripheral smears that showed schistocytes were included in the study, irrespective of age and gender of the patient. One hundred and fifty-seven PBSs with schistocytes were included after calculating sample size from previous studies (z = 137).[2] Blinded review of the Leishman-stained slides were performed independently by two observers with similar levels of expertise in diagnostic hematology, under 1,000× magnification (oil immersion) using ICSH recommendations.[1] The schistocytes were quantified by counting 1,000 RBCs and presented as a percentage. The other RBC morphologic forms such as macrocytes, microcytes, and polychromatophilic cells and poikilocytes such as target cells, spherocytes, and elliptocytes were identified. These cells too were quantified after counting 1000 RBCs, expressed as a percentage and graded on a scale of 1+ to 3 +, based on the morphologic grading table as per ICSH recommendations.[5] Clinical information, diagnosis, and other relevant laboratory investigations were obtained from the laboratory information system. Cases without clinical data, hemolyzed samples, and poor film quality smears were excluded from the study.
Statistical analysis was done using SPSS—Statistical Package for the Social Sciences—software, version 22. Continuous data were represented as mean and standard deviation. Mann–Whitney U test was used as test of significance to identify the mean difference between the two qualitative variables. Chi-squared test was used as test of significance for qualitative data. A p-value of less than 0.05 was statistically significant. Data plotting was done using Bland-Altman chart to compare the measurements.
Results
Patients' age ranged from newborn to 84 years, with majority of cases lying between 51 and 60 years (►Table 1) and with male-to-female ratio of 2:1.
| Age | Number | Percentage |
|---|---|---|
| Neonates | 21 | 13.4 |
| 1 month to 10 years | 17 | 10.8 |
| 11–20 years | 12 | 7.7 |
| 21–30 years | 10 | 5.9 |
| 31–40 years | 14 | 9.0 |
| 41–50 years | 15 | 9.6 |
| 51–60 years | 30 | 19.2 |
| 61–70 years | 27 | 17.3 |
| 71–80 years | 9 | 5.8 |
| > 80 years | 2 | 1.3 |
Patients were categorized according to diagnosis (n = 157). Five cases were diagnosed as TMA and remaining 152 cases belonged to the non-TMA group.
Patients with TMA included three cases of disseminated intravascular coagulopathy and two cases of TTP, of which one was idiopathic TTP and the other was systemic lupus erythematosus (SLE) complicated with TTP (►Fig. 1A). All five cases showed schistocyte percentage of more than 1% as shown in ►Table 2. All five cases showed schistocyte as the major RBC morphologic abnormality.

- (A) Schistocytes (arrows) in case of systemic lupus erythematosus complicated with thrombotic thrombocytopenic purpura. (B) Schistocytes (arrows) in nonthrombotic microangiopathy case with other poikilocytes–elliptocytes (arrowhead) and target cell (); (Leishman stain; 1,000×).
| Diagnoses | No. of cases | Schistocyte percentage | |||||
|---|---|---|---|---|---|---|---|
| 0–1% | 1–3% | > 3% | |||||
| Count | % | Count | % | Count | % | ||
| DIC | 3 | 0 | 0 | 3 | 100 | 0 | 0.0 |
| TTP | 2 | 0 | 0 | 1 | 50.0 | 1 | 50.0 |
| Preterm | 14 | 7 | 50.0 | 6 | 42.9 | 1 | 7.1 |
| Term neonates | 7 | 4 | 57.1 | 3 | 42.9 | 0 | 0 |
| Hematological malignancy | 4 | 4 | 100 | 0 | 0 | 0 | 0 |
| Iron deficiency anemia | 2 | 2 | 100 | 0 | 0 | 0 | 0 |
| Nutritional anemia | 18 | 18 | 100 | 0 | 0 | 0 | 0 |
| Megaloblastic anemia | 6 | 6 | 100 | 0 | 0 | 0 | 0 |
| Chronic liver disorder | 14 | 10 | 71.4 | 4 | 28.5 | 0 | 0 |
| Lung disease | 6 | 5 | 83.3 | 1 | 16.7 | 0 | 0 |
| Heart disease | 9 | 6 | 66.7 | 3 | 33.3 | 0 | 0 |
| Chronic renal disorder | 18 | 13 | 72.2 | 5 | 29.4 | 0 | 0 |
| Infection | 44 | 35 | 79.5 | 8 | 18.2 | 1 | 2.3 |
| Autoimmune disease | 3 | 2 | 66.7 | 1 | 33.3 | 0 | 0 |
| Malignancy | 7 | 6 | 85.7 | 1 | 14.3 | 0 | 0 |
Abbreviations: DIC, disseminated intravascular coagulopathy; TTP, thrombotic thrombocytopenic purpura.
Non-TMA group comprised predominantly of infectious conditions (44), many admitted with Coronavirus Disease-2019 infection (32), and a few cases complicated with sepsis. The other major non-TMA categories included chronic renal failure (18), nutritional anemias (18), preterm infants (14), and nonhematological malignancies (7), as shown in ►Table 2.
Of the 152 non-TMA cases, 118 had schistocytes less than 1%. The remaining 34 cases had schistocyte percentage more than 1%, which included 9 cases of infection in sepsis, 7 preterm infants, 5 cases of chronic renal failure, 4 cases of chronic liver disease, 3 term neonates, 2 cases of ischemic heart disease, 1 case with prosthetic heart valve, and 1 case each of SLE, nonhematologic malignancy (gastric adenocarcinoma), and chronic lung disease. In all these 34 cases, other poikilocytes such as elliptocytes, target cells, teardrop cells, or acanthocytes were present (►Fig. 1B) ranging from 2+ to 3+ as per ICSH morphologic grading system. Thus, the schistocytes accounted for a small fraction of the poikilocytes.
With respect to reproducibility of schistocyte count by application of ICSH criteria, the mean of difference in schistocyte percentage between Observer 1 and Observer 2 was 0.19 ± 1.96 (0.59); 95% confidence interval (CI) = –0.966 to 1.346. As the 95% CI was close to 0, the agreement between the two observers in schistocyte percentage was excellent, as shown in ►Fig. 2. Thus, the schistocyte percentage between the two independent observers showed good agreement, in terms of both statistical significance and quantification of schistocytes (►Tables 3 and ►4).
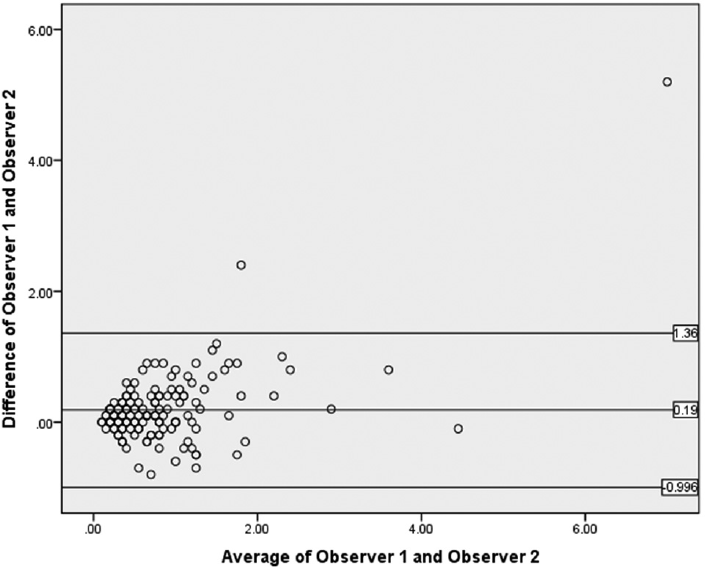
- Bland-Altman plot showing agreement between Observer 1 and Observer 2.
| Diagnosis | Schistocyte % by Observer 1 | Schistocyte % by Observer 2 | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Median | Mean | SD | Median | |
| TMA | 2.68 | 3.87 | 1.00 | 1.88 | 1.43 | 1.30 |
| Non-TMA | 0.86 | 0.73 | 0.70 | 0.69 | 0.60 | 0.60 |
| p-Value | < 0.001* | < 0.001* | ||||
Abbreviations: SD, standard deviation; TMA, thrombotic microangiopathy.
* statistically significant as p value is <0.05
| Mean | SD | Median | Minimum | Maximum | |
|---|---|---|---|---|---|
| Schistocyte % by Observer 1 | 0.92 | 1.01 | 0.70 | 0.10 | 9.60 |
| Schistocyte % by Observer 2 | 0.73 | 0.67 | 0.60 | 0.10 | 4.50 |
| Average of Observer 1 and Observer 2 | 0.83 | 0.81 | 0.65 | 0.10 | 7.00 |
| Difference of Observer 1 and Observer 2 | 0.19 | 0.59 | 0.10 | –0.80 | 5.20 |
Abbreviation: SD, standard deviation.
Discussion
Schistocytes are being increasingly utilized as the hallmark of TMA. As per ICSH guidelines, the presence of more than 1% schistocytes in peripheral smear is of clinical relevance as opposed to other poikilocytes (>20%). However, it lacked specific morphologic criteria. Hence, in 2008 International Schistocyte Working Group came together and put forth the criteria in 2012.[1–3] Schistocytes are described as red cell fragments that are always smaller than intact RBCs having sharp angles, straight borders/helmet cells, small crescents, and keratocytes. Furthermore, microspherocytes are considered significant and included in schistocyte count if they are found in association with the above-described forms.[1] TMAs encompass a spectrum of clinical syndromes of different etiopathogenetic pathways resulting in excessive activation of platelets that deposit as thrombi in small blood vessels. The syndromes include TTP, hemolytic uremic syndrome, disseminated intravascular coagulopathy, and hemolysis with elevated liver enzymes and low-platelet syndrome, with the common denominator being schistocytes and polychromatophilia (reticulocytosis) in the PBS and negative direct antiglobulin (Coombs) test.[2–4]
TTP is a clinicopathologic syndrome of unknown etiology and pathogenesis comprises of microangiopathic hemolytic anemia, thrombocytopenia, and widespread hyaline thrombi in arterioles and capillaries.[3,6,7] Diagnostic pentad included microangiopathic hemolytic anemia, thrombocytopenia, neurologic deficits, renal abnormalities, and fever, which are not always seen in all patients; hence, it was reduced to triad and now to a diad of microangiopathic hemolytic anemia and thrombocytopenia. The main reason for this shift was the observation that many patients presented with diad syndrome before advancing to triad and pentad.[3,6–8] Early diagnosis and plasma exchange therapy with fresh frozen plasma is warranted.[2–4] However, diagnosis depends mainly on clinical suspicion and laboratory tests. Confirmatory diagnosis of TTP requires sophisticated and time-consuming assays measuring “a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13” (ADAMTS13) activity or presence of neutralizing antibodies. Hence, manual peripheral smear quantification of schistocytes plays a vital role in diagnosis and appropriate management.[6] SLE is a multisystem disorder and can be complicated with TTP requiring early intervention. Further, SLE is not associated with low ADAMTS13 activity, thus emphasizing the role of peripheral smear schistocyte estimation in the early diagnosis.[8] The finding of schistocytes in patients with TTP and other TMAs is variably reported to be in excess of 0.5 to 1.0%, with more than or equal to 1% commonly referenced as the threshold for clinical significance.[6,7] Thus, ICSH Schistocyte Working Committee recommended that a schistocyte percentage above 1% after counting 1,000 RBCs on peripheral smear is required for the diagnosis of TMA.[1] In this study with strict application of ICSH criteria, schistocytes accounted for more than 1% in all cases of TMA (n = 5) and were statistically significant similar to studies by Huh et al and Schapkaitz and Mezgebe.[2,3]
There are conditions other than TMA where schistocytes are seen in large numbers. The non-TMA conditions in this study included cases admitted for infectious diseases, chronic renal disorders, chronic liver disorders, lung diseases, heart diseases, and nutritional/megaloblastic anemias (n = 152). These findings were similar to studies by Huh et al and Schapkaitz and Mezgebe.[2,3] However, in the non-TMA cases, schistocytes were only a minor component of a more global anisopoikilocytosis similar to other studies.[2,3] Thus, schistocytes should be considered significant only if it represents the main morphologic RBC abnormality in the peripheral smear examination.[1,2,4]
Of the 152 non-TMA cases associated with schistocytes, 34 cases had schistocyte percentage of more than 1%. Preterm and term neonates comprised 10 cases (10/34). Schistocytes are considered significant if it accounts for more than 1% in full-term neonates and more than 5% in preterm neonates.[3] Higher percentage of schistocytes in preterm results from liver immaturity.[2] The current study also showed agreement with higher percentage of schistocytes in preterm neonates (n = 7). The term neonates (n = 3) in the study were in sepsis, which included one case of necrotizing enterocolitis.[9]
Among anemias, megaloblastic anemia with hemolysis is often misdiagnosed as TMA due to pancytopenia with schistocytes. Pathogenesis of pseudo-TMA involves homocysteine-induced endothelial injury and dysfunction. Hyperhomocysteinemia triggers activation of coagulation cascade and alters the endothelium. In addition, macrocytic erythrocytes resulting from vitamin B12 deficiency are associated with reduced deformability and fragmentation as they flow through the microvasculature.[10] Further, a study by Biswal et al on schistocytes in megaloblastic anemia associated with hemolysis was associated with decreased mean corpuscular volume and a schistocyte percentage of more than 1% in 29 out of 30 cases.[10] However, this study included six cases of megaloblastic anemia, of which five had raised mean corpuscular volume (102–125 fl) and one had normal mean corpuscular volume, with mean schistocyte percentage of 0.5%.
Further, 9/34 patients admitted for infectious condition in sepsis as evidenced by clinical and laboratory criteria such as elevated serum procalcitonin/C-reactive protein had schistocytes in excess of 1% among the non-TMA cases. Sepsis is a serious medical condition associated with hemostatic abnormalities ranging from isolated thrombocytopenia and/or subclinical activation of coagulation cascade to precipitation of acute disseminated intravascular coagulopathy.[11]
Mechanical trauma to RBCs resulting from cardiac valvular disease and malfunctioning prosthetic valves is also associated with hemolytic anemia presenting with peripheral blood schistocytes. This study recorded one case of prosthetic heart valve with schistocyte more than 1%.[4]
Also, 5/34 patients admitted with chronic renal disorder had schistocyte more than 1% similar to a study by Schapkaitz and Mezgebe.[2] A study conducted by Shastry and Belurkar titled “The spectrum of red blood cell parameters in chronic kidney diseases: A study of 300 cases” demonstrated that the presence of schistocytes and spherocytes was mostly seen with advanced stage of chronic kidney disease. The raised serum urea level results in increased expression of phosphatidyl serine on outer leaflet of the RBC membrane making them prone to hemolysis.[12]
Chronic liver disorders accounted for 4/34 cases with schistocytes more than 1% on PBS. Rare schistocytes were reported in a case of spur cell anemia arising in a cirrhotic liver.[13] Chronic disseminated intravascular coagulation is of concern in cirrhotic livers. Alcohol-related liver diseases are associated with hemolysis owing to alcohol toxicity on red cell membranes. Further, liver plays a key role in lipid metabolism; thus, alteration of lipid homeostasis in chronic liver disorders results in increased cholesterol to phospholipid on RBC membrane, which favors hemolysis.[13]
Cancer-related microangiopathy is associated with thrombocytopenia and clinical or pathological evidence of microvascular thrombosis in various organs.[4] This study had 7/152 nonhematologic malignancies with schistocytes, of which 1 case had thrombocytopenia and schistocyte more than 1%.
Present-day automated hematology analyzers flag fragmented red cells based on selected threshold.[1,2] A study by Saigo et al[14] showed a good correlation of fragmented red cells with manual schistocyte identification.[1] However, in this study, fragmented red cells were underestimated (7/157 smears) similar to a study by Banno et al.[1,15] ICSH recommends all samples with positive automated fragmented red cell count and macrocytic samples with negative fragmented red cell count be manually reviewed.[1]
Furthermore, with strict application ICSH criteria, the interobserver reproducibility of schistocyte percentage showed good agreement between the two independent observers. A study by Noutsos et al comparing ICSH method with another proposed method by the study group showed moderate interobserver strength of agreement.[8]
Conclusion
Schistocytes provide an insight into various etiopathogenetic roles to explain their appearance in peripheral smear. Schistocytes, when present as the major morphologic abnormality on smear examination, amounting for more than 1% in adults favor the diagnosis of TMA. However, when seen concurrently with other poikilocytes, is suggestive of non-TMA conditions and need not be quantified. Strict application of ICSH guidelines facilitates good reproducibility and reduces interobserver bias making it a robust marker in early diagnosis of TMA.
Ethical Approval
Ethical clearance was obtained from the Institutional Ethics Committee, Kempegowda Institute of Medical Sciences, dated 10/7/2021, bearing the reference number KIMS/IEC/A049/M/2021.
Details of Earlier Presentation
Presented as online oral paper in Karnataka Chapter-Indian Association of Pathologists and Microbiologists—KCIAPM—annual state conference held in September 2021.
Authors' Contributions
T.S. helped in conception and design of the study, acquisition of data, interpretation of data, and drafting of the article. C.M. was involved in conception and design of the study, analysis and interpretation of data, critical revision of the draft, and final approval of the version. G.B.R. helped in conception and design of the study, analysis and interpretation of data, critical revision of the draft, and final approval of the version.
Conflict of Interest
None declared.
Funding
Nil.
References
- International Council for Standardization in Haematology (ICSH). ICSH recommendations for identification, diagnostic value, and quantitation of schistocytes. Int J Lab Hematol. 2012;34(02):107-116.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical significance of schistocytes: a prospective evaluation of the International Council for Standardization in Hematology schistocyte guidelines. Turk J Haematol. 2017;34(01):59-63.
- [CrossRef] [PubMed] [Google Scholar]
- Harrison's Principles of Internal Medicine. (20th). New York: McGraw-Hill, Health Professions Division; 2022. p. :2148-2165.
- [Google Scholar]
- Microscopic schistocyte determination according to International Council for Standardization in Hematology recommendations in various diseases. Int J Lab Hematol. 2013;35(05):542-547.
- [CrossRef] [PubMed] [Google Scholar]
- ICSH recommendations for the standardization of nomenclature and grading of peripheral blood cell morphological features. Int J Lab Hematol. 2015;37(03):287-303.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombotic Thrombocytopenic Purpura, Hemolytic-Uremic Syndrome, and Related Disorders. In: Wintrobe's Clinical Hematology (14th). Europe: Wolters Kluwer Health Pharma Solutions; 2018. p. :3434-3495.
- [Google Scholar]
- Microangiopathic haemolytic anaemia resembling thrombotic thrombocytopenic purpura in systemic lupus erythematosus: the role of ADAMTS13. Rheumatology (Oxford). 2011;50(05):824-829.
- [CrossRef] [PubMed] [Google Scholar]
- An evaluation of existing manual blood film schistocyte quantitation guidelines and a new proposed method. Pathology. 2021;53(06):746-752.
- [CrossRef] [PubMed] [Google Scholar]
- Neonates with suspected microangiopathic disorders: performance of standard manual schistocyte enumeration vs. the automated fragmented red cell count. J Perinatol. 2019;39(11):1555-1561.
- [CrossRef] [PubMed] [Google Scholar]
- Schistocytosis in megaloblastic anemia masquerading peripheral blood picture as maha (microangiopathic hemolytic anemia) and decrease mean corpuscular volume. Indian J Pathol Oncol. 2020;7(03):357-361.
- [CrossRef] [Google Scholar]
- Sepsis-associated disseminated intravascular coagulation and thromboembolic disease. Mediterr J Hematol Infect Dis. 2010;2(03):e2010024.
- [CrossRef] [PubMed] [Google Scholar]
- The spectrum of red blood cell parameters in chronic kidney disease: a study of 300 cases. J Appl Hematol. 2019;10:61-66.
- [CrossRef] [Google Scholar]
- The diagnosis is in the smear: a case and review of spur cell anemia in cirrhosis. Case Rep Hematol. 2021;2021 8883335
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness of automatic detection of fragmented red cells using a hematology analyzer for diagnosis of thrombotic microangiopathy. Clin Lab Haematol. 2002;24(06):347-51.
- [CrossRef] [PubMed] [Google Scholar]
- Quantification of red blood cell fragmentation by the automated hematology analyzer XE-2100 in patients with living donor liver transplantation. Clin Lab Haematol. 2005;27(05):292-296.
- [CrossRef] [PubMed] [Google Scholar]





