Translate this page into:
Urine Albumin Excretion as a Marker of Acute Glycemic Changes in Isolated Postprandial Hyperglycemia
Address for correspondence: Dr. Alagilawada S Shilpasree, E-mail: shilpasree2007@rediffmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Postprandial hyperglycemia is a major risk factor for the development of cardiovascular diseases (CVDs), and Most of the times it occurs in patients with normal glycemic control diagnosed by fasting blood glucose (FBG) and glycated hemoglobin levels. Urine albumin excretion (UAE) is an independent predictor of CVD risk.
Aim:
To estimate UAE in isolated postprandial hyperglycemia (IPPHG) patients and to assess the relationship of UAE with FBG and postprandial blood glucose (PPBG) levels.
Settings and Design:
A cross-sectional study was carried out in 318 patients with Type II diabetes in the age group 30–60 years for 6 months.
Materials and Methods:
Patients were divided into five groups based on their FBG and PPBG values. UAE and lipid profile were measured in all the groups.
Statistical Analysis:
UAE and lipid profile in different groups were compared using ANOVA. Regression analysis was used to predict the variation of UAE with FBG, PPBG, and total cholesterol (TC).
Results:
Patients with IPPHG had significantly higher albumin excretion compared to normoglycemia (NG) group [P < 0.0001]. In impaired glucose tolerance and isolated fasting hyperglycemia groups, it did not differ significantly from NG group [P = 0.206 and P = 0.173]. Lipid profile did not show any significant difference between the groups. On regression analysis, PPBG but not FBG or TC correlated positively with UAE.
Conclusion:
UAE is easy, less expensive, and Widely available method done on spot urine samples which predicts the acute glycemic changes and increased risk of developing CVDs in patients with IPPHG.
Keywords
Hyperglycemia
impaired glucose tolerance
normoglycemia
urine albumin excretion
INTRODUCTION
Diabetes is a major health problem in developed as well as in developing countries Due to its association with high cardiovascular mortality. Cardiovascular diseases (CVDs) are the cause of death in approximately 65% of diabetic patients. To make the matter worse, diabetics who develop CVD will have a worse prognosis than the CVD patients without diabetes.[1] Although various modalities are available for the diagnosis of diabetes mellitus (DM), the most commonly used one is fasting blood glucose (FBG) due to its low cost, wide availability, and easy reproducibility. According to the American Diabetic Association, glycated hemoglobin (HbA1c) (threshold >6.5%) can also be used to diagnose diabetes. However, the use of HbA1c is not widely acceptable, especially in developing countries due to its high cost and limited availability of certified methods.[2]
Until recently, FBG and HbA1c were considered as valid markers of overall glycemic control and were assessed routinely. Many studies now have suggested the role of postprandial blood glucose (PPBG) excursions in the overall glycemic load and development of microvascular and macrovascular complications of diabetes. Postprandial hyperglycemia in impaired glucose tolerance (IGT) and diabetic subjects is a more powerful risk factor of CVD than fasting hyperglycemia.[3] Isolated postprandial hyperglycemia (IPPHG) is defined as FBG in nondiabetic range (<126 mg/dl) and PPBG in diabetic range (>200 mg/dl).[4] The prevalence varies widely from 20% to 40%.[56] Many times, it occurs in patients with good glycemic control diagnosed by HbA1c levels. Even though the exact mechanism behind the risk of CVDs and postprandial glucose excursions is not clear, it may be attributed to sudden increase in blood glucose levels causing oxidative stress through the Pathways regulated by NF-κβ leading to DNA damage and endothelial dysfunction (due to reduced generation or availability nitric oxide [NO]) coupled with low-density lipoprotein (LDL) oxidation, progressing to low-grade systemic inflammation and atherosclerosis.[78]
Microalbuminuria is defined as the urinary albumin excretion 30–300 mg/day or 20–200 mg/L[9] and it is shown as an independent predictor of CVD risk and mortality in hypertensive, diabetic, as well as in normal population. Many studies have shown that the association of urinary albumin excretion and cardiovascular mortality begins at a very low threshold even below the range of microalbuminuria and extends beyond the range. Furthermore, any decrease in albuminuria is strongly related to decrease in the risk of CVDs.[1011] Even though the association of microalbuminuria and CVD is not clear, it is postulated that chronic inflammation and endothelial dysfunction contribute to the underlying pathogenesis.[9] Since postprandial hyperglycemia induces endothelial dysfunction and vascular failure due to acute glycemic spikes resulting after meals, it is possible that IPPHG patients have more urine albumin excretion (UAE) than the patients with fasting hyperglycemia, combined fasting and postprandial hyperglycemia (CFPPHG), and subjects with normoglycemia (NG). Moreover, there is no systematic study showing the exact contribution of FBG or PPBG to UAE and the possible association of postprandial hyperglycemia with increased cardiovascular mortality. Hence, the aim of our study is to assess the urinary albumin excretion in postprandial hyperglycemia patients and to compare it with fasting hyperglycemia, CFPPHG, and normal glucose tolerance subjects.
MATERIALS AND METHODS
A cross-sectional study was carried out in patients with Type II DM attending outpatient department of SDM College of Medical Sciences and Hospital for 6 months. After obtaining the ethical clearance from the institutional ethical committee, a total number of 318 previously diagnosed Type II DM patients in the age group of 30–60 years were included in the study. Patients with gestational DM, renal diseases, hypertension, history of CVD, and who are on treatment with angiotensin-converting enzyme (ACE) inhibitors were excluded from the study. Informed consent was obtained from all the participants.
About 5 ml of blood was drawn under aseptic precautions after overnight fasting of 12 h and serum separated by centrifugation used for the analysis. The estimation of FBG was done by hexokinase method. Lipid profile was estimated by enzymatic methods. Total cholesterol (TC) was estimated by cholesterol oxidase-peroxidase method, LDL-cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) by direct methods, and triglycerides (TGs) by glycerol phosphate oxidase-peroxidase method. Blood urea was measured by enzymatic urease method and serum creatinine by Jaffe's kinetic method. A spot urine sample was collected in a separate urine container and used for the estimation of UAE by immunoturbidimetric method. All estimations were carried out on fully autoanalyzer (Siemens dimension RxL Max). Patients were advised to have regular meals and come after 2 h. A postprandial sample was collected after 2 h and used for the estimation of PPBG. Patients were divided into five groups based on their FBG and PPBG values.
Group 1: NG, FBG is <110 mg/dl and PPBG is <140 mg/dl.
Group 2: IGT, FBG is 110–125 mg/dl and PPBG is 140–199 mg/dl.
Group 3: Isolated fasting hyperglycemia (IFHG), FBG is ≥126 mg/dl and PPBG is <200 mg/dl.
Group 4: IPPHG, FBG is <126 mg/dl and PPBG is ≥200 mg/dl.
Group 5: CFPPHG, FBG is ≥126 mg/dl and PPBG is ≥200 mg/dl.
Statistical analysis
Statistical analysis was done using IBM SPSS 20 version, IBM corporation, Route 100, Somers, New York- 10589, USA. Unpaired t-test was used to compare the mean UAE, TC, LDL-C, HDL-C, and TGs in NG group with IGT, IFHG, IPPHG, and CFPPHG groups. P < 0.05 was considered statistically significant. Multiple regression analysis was done to predict the variation of UAE with FBG, PPBG, and TC. ANOVA was used to compare the mean UAE in different groups.
RESULTS
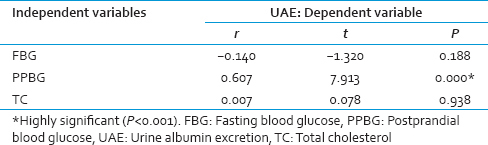
A total 282 subjects were enrolled in the study after applying above-mentioned exclusion criteria. The mean age of participants was 50 ± 9.5 years. Descriptive information of the subjects is given in Table 1. Subjects were divided into five groups based on their FBG and PPBG levels. There was no significant difference in the age between different groups. Mean FBG, PPBG, UAE and lipid profile of different groups are given in Table 2. Mean FBG, PPBG, and UAE in each group are shown in Figure 1. PPBG elevation in different groups is shown in Figure 2. Relation of UAE with mean PPBG-FBG is given in Figure 3. UAE in IGT group and IFHG group did not show any significant difference from NG group (P = 0.206 and P = 0.173). Whereas, in IPPHG group and CFPPHG group, it was higher than the NG group and the results were statistically significant (P < 0.001). One-way ANOVA showed that UAE differs significantly between the groups, F = 6.793 and P = 0.000. Tukey's post hoc analysis revealed that UAE is significantly higher in IPPHG group when compared to NG and IFHG groups (P = 0.0002, 0.0426) and in CFPPHG group when compared to NG group (P = 0.0008). On the other hand TC, HDL-C and TG did not show any significant difference between the groups. Multiple regression analysis using UAE as dependent variable FBG, PPBG, and TC as independent variables is given in Table 3. UAE correlated significantly with PPBG levels (P < 0.001) but not with FBG (P = 0.188) and TC (P = 0.938) levels.


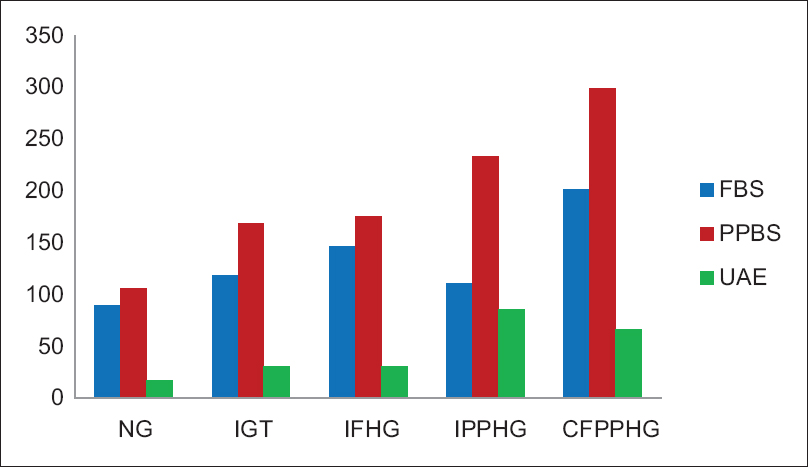
- Fasting blood glucose, postprandial blood glucose (mg/dl), and urine albumin excretion (mg/L) in different groups
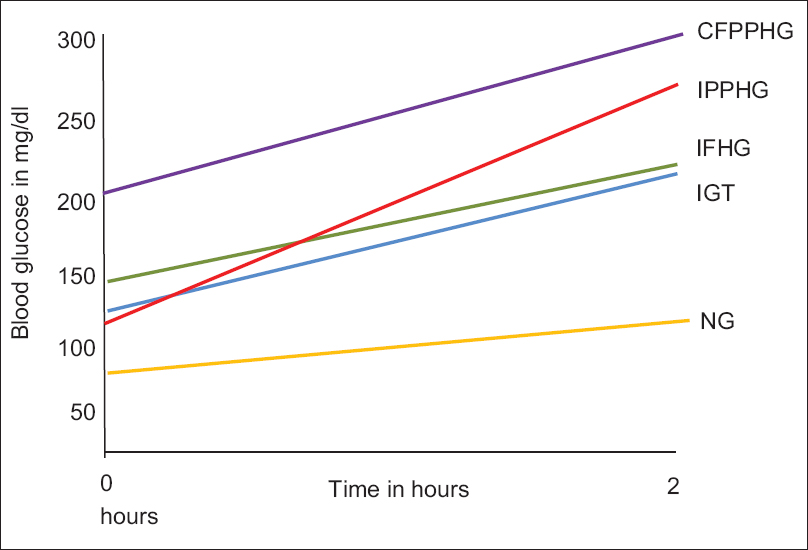
- Blood glucose elevation following meals in different groups
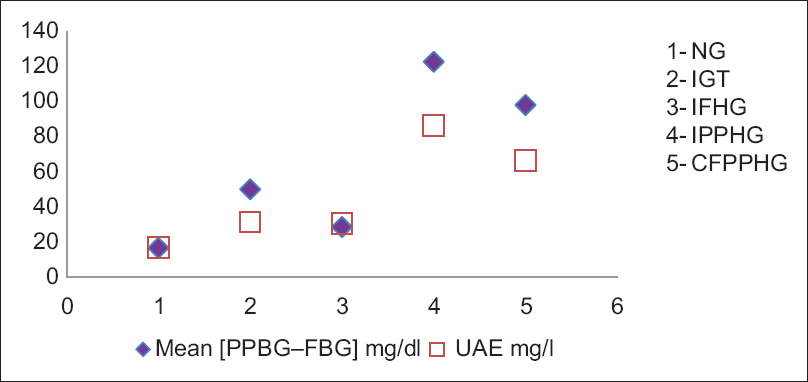
- Relation of urine albumin excretion with mean (postprandial blood glucose-fasting blood glucose) in different groups
DISCUSSION
Recently, many studies have shown the elevated PPBG as major risk factor for CVD and all-cause mortality independent of FBG and HbA1c levels,[121314] and UAE is widely recognized strong and independent predictor of CVD risk in diabetic as well as nondiabetic individuals.[111516] This association of UAE and CVD risk is independent of traditional cardiovascular risk factors. It begins well below the conventional microalbuminuria cutoff and increases with increasing UAE.[1718] Microalbuminuria can be a cause or consequence of systemic vascular dysfunction. Endothelial dysfunction may contribute to the pathogenesis of microalbuminuria by altering glomerular basement membrane structure and glomerular filtration pressure, or they both can arise from the common underlying cause. Reduction in the UAE is associated with reduced risk of adverse cardiovascular events.[19] Prevention of renal and vascular endstage disease intervention trial from a large cohort of 1439 subjects showed significant reduction in cardiovascular events in patients with microalbuminuria when treated with ACE inhibitor fosinopril. Treatment led to 26% reduction in UAE and 40% reduction in primary cardiovascular endpoints.[20] In our study, we estimated UAE in IPPHG and compared it with NG group. Patients with IPPHG had markedly higher UAE, in contrast to IGT and IFHG groups where UAE did not differ significantly compared to NG group. Patients with CFPPHG also had higher UAE than NG group, but it was less compared to IPPHG patients. This can be attributed to acute increase in blood glucose levels in the form of sharp glycemic spikes following meals in patients with IPPHG leading to oxidative stress and generation of free radicals which is the main contributing factor for initiating endothelial dysfunction leading to microalbuminuria.
On the other hand, patients with CFPPHG who have higher baseline glucose concentration experience low magnitude glycemic spike following meal, leading to less UAE compared to IPPHG patients. This indirectly supports the fact that it is not the baseline glucose concentration but the magnitude of glycemic spike resulting postmeal that directly influences the UAE and cardiovascular mortality. Hence, measuring UAE is an easier way to indicate the acute glycemic changes. Sudden elevation in the plasma glucose levels triggers tissue responses such as generation of oxygen free radicals, LDL oxidation, and endothelial dysfunction due to decreased production of NO and lack of response to NO. It is also observed that increased production of collagen from the mesangial cell, activation of blood coagulation that is likely to cause thrombosis, increase in blood pressure, increase in the circulating levels of intracellular adhesion molecule-1, increase production of plasma interleulin-6, interleukin-18, and tumor necrosis factor-a, thus activating one of the first stages of the atherogenic process, low-grade systemic inflammation.[378] Direct evidence to this is obtained from the studies which showed acute hyperglycemia induced by meals increased the production of free radicals, decreased the level of antioxidants, and increased the susceptibility of LDL to oxidation.[2122] In a study conducted by Ge et al., intermittent glucose excursions induced more oxidative stress and generated reactive oxygen species compared to constant high glucose.[23]
The main goal of treating diabetes patients is to establish and maintain good glycemic control to prevent the microvascular and macrovascular complications. HbA1c is well established and considered the gold standard for monitoring overall glycemic control resulting from FBG and PPBG levels. In our study, we have not included HbA1c levels because it is not clear whether the HbA1c levels are influenced by PPBG values in the same way as FBG values and the extent to which HbA1c reflects the acute glycemic spikes. Recently, many studies have demonstrated the better correlation of HbA1c with preprandial blood glucose or FBG levels rather than PPBG levels.[5] In a study conducted by Saab et al., HbA1c correlated better with overnight glucose levels compared to mean morning, afternoon, and evening blood glucose levels.[24] This can be attributed to the fact that more time of the day is spent in interprandial and nocturnal periods rather than postprandial phase. As a result, average blood glucose reflected by HbA1c is more a function of interprandial or nocturnal glucose levels but not the PPBG. Monnier et al. have shown that contribution of PPBG is predominant in patients with moderate DM (HbA1c <8.4) but the contribution of FBG increases as the diabetes worsens (HbA1c >8.4).[25] Hence, monitoring only FBG or HbA1c may not prove satisfactory in controlling the complications of diabetes unless PPBG is not addressed effectively. This is in consistent with the United Kingdom prospective diabetes study which revealed reduction in HbA1c by intensive blood glucose control had no effect on reducing CVD mortality.[26] On the other hand, when PPBG was controlled, better cardiac outcome was observed.
PPBG is also associated with carotid artery intima-media thickness (CIMT) and Esposito et al. documented reduction in PPBG significantly reduced CIMT in 52% patients with Type II diabetes and improved mortality.[27] Many treatment modalities are available including short-acting sulfonylureas, rapid-acting human insulin, glucosidase inhibitors such as acarbose and rapid-onset/short-duration insulinotropic agent, repaglinide to specifically target PPBG levels.[28] The STOP-NIDDM trial has provided the evidence of reduction in not only the myocardial infarction and CV events but also the other manifestations of diabetes in patients with IGT treated with acarbose.[29] Hence, it is important to identify this subset of patients with IPPHG who need extensive investigation, monitoring, and treatment strategy directed specifically against PPBG to improve the outcome.
For the first time in our study, UAE is used as an indicator of the acute glycemic changes occurring in IPPHG leading to endothelial dysfunction and vascular failure contributing to cardiovascular mortality. Even though many studies have documented the role of postprandial hyperglycemia in macrovascular complications of diabetes and microalbuminuria as independent predictor of CVD risk, no studies have shown the relationship of UAE with FBG or PPBG. In our study, we have reported the good correlation of UAE with PPBG but not the FBG indicating the need to include PPBG levels and UAE along with FBG and HbA1c to assess the glycemic variations and monitor the patients with Type II DM.
CONCLUSION
Measuring UAE is relatively easy, less expensive, Widely available and done on spot urine samples. Increased UAE in patients with IPPHG indicates that it is not the baseline blood glucose but the acute glycemic changes in the form of sharp glycemic spikes leading to endothelial dysfunction and low-grade systemic inflammation which further contributes to increased risk of CVDs. For the first time, our study has demonstrated the correlation of UAE with PPBG but not with FBG. Hence, it is necessary to include PPBG and UAE levels to identify the subset of patients with IPPHG who most of the times have optimum glycemic control as measured by FBG and HbA1c levels and target the treatment to specifically reduce the PPBG levels to improve the mortality and morbidity associated with cardiovascular events.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Diabetes and cardiovascular disease: A statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134-46.
- [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes – 2014. Diabetes Care. 2014;37(Suppl 1):S14-80.
- [Google Scholar]
- Postprandial hyperglycemia and diabetes complications: Is it time to treat? Diabetes. 2005;54:1-7.
- [Google Scholar]
- Factors responsible for development from normal glucose tolerance to isolated postchallenge hyperglycemia. Diabetes Care. 2003;26:1211-5.
- [Google Scholar]
- Plasma glucose levels throughout the day and HbA(1c) interrelationships in Type 2 diabetes: Implications for treatment and monitoring of metabolic control. Diabetes Care. 2001;24:2023-9.
- [Google Scholar]
- Isolated postprandial hyperglycemia in Type 2 diabetic patients in a Nigerian Tertiary Health Center. Indian J Endocrinol Metab. 2012;16:604-8.
- [Google Scholar]
- Postprandial glycemia and cardiovascular disease in diabetes mellitus. Arq Bras Endocrinol Metabol. 2007;51:212-21.
- [Google Scholar]
- Postprandial hyperglycemia as an etiological factor in vascular failure. Cardiovasc Diabetol. 2009;8:23.
- [Google Scholar]
- Microalbuminuria and risk of cardiovascular diseases in patients with diabetes and hypertension. Biochem Med. 2008;18:25-34.
- [Google Scholar]
- Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms. J Am Soc Nephrol. 2006;17:2106-11.
- [Google Scholar]
- A prospective study of microalbuminuria and incident coronary heart disease and its prognostic significance in a British population: The EPIC-Norfolk study. Am J Epidemiol. 2004;159:284-93.
- [Google Scholar]
- Postprandial glycaemic excursions and cardiovascular risk. J Indian Med Assoc. 2011;109:912, 917-20.
- [Google Scholar]
- Risk factors for myocardial infarction and death in newly detected NIDDM: The diabetes intervention study, 11-year follow-up. Diabetologia. 1996;39:1577-83.
- [Google Scholar]
- DECODE Study Group, the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397-405.
- [Google Scholar]
- Arterial hypertension, microalbuminuria, and risk of ischemic heart disease. Hypertension. 2000;35:898-903.
- [Google Scholar]
- Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in Type 2 diabetes: Progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51:1157-65.
- [Google Scholar]
- Albumin excretion rate and cardiovascular risk: Could the association be explained by early microvascular dysfunction? Diabetes. 2005;54:1816-22.
- [Google Scholar]
- Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421-6.
- [Google Scholar]
- Microalbuminuria in type 2 diabetes and hypertension: A marker, treatment target, or innocent bystander? Diabetes Care. 2008;31(Suppl 2):S194-201.
- [Google Scholar]
- Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110:2809-16.
- [Google Scholar]
- Association of postprandial hyperglycemia with in vitro LDL oxidation in non-smoking patients with type 1 diabetes – a cross-sectional study. Rev Diabet Stud. 2005;2:157-64.
- [Google Scholar]
- Meal-induced oxidative stress and low-density lipoprotein oxidation in diabetes: The possible role of hyperglycemia. Metabolism. 1999;48:1503-8.
- [Google Scholar]
- Effects of intermittent high glucose on oxidative stress in endothelial cells. Acta Diabetol. 2010;47(Suppl 1):97-103.
- [Google Scholar]
- Contributions of overnight glycemia to the overall hyperglycemia of type 2 diabetic patients: Variations with HbA1c levels. J Diabetes Sci Technol. 2014;8:619-20.
- [Google Scholar]
- Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: Variations with increasing levels of HbA (1c) Diabetes Care. 2003;26:881-5.
- [Google Scholar]
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-53.
- [Google Scholar]
- Campanian Postprandial Hyperglycemia Study Group. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110:214-9.
- [Google Scholar]
- Postprandial hyperglycemia and glycemic variability: Should we care? Diabetes Care. 2011;34(Suppl 2):S120-7.
- [Google Scholar]





