Translate this page into:
Utility of a novel chromogenic medium as a screening method in the detection of carbapenemase producing Enterobacteriaceae
Address for correspondence: Dr. Anusha Chaturvedi, Department of Microbiology, M.S. Ramaiah Medical College, MSRIT Post, Bangalore 560 054, Karnataka, India. E-mail: anusha_chaturvedi@yahoo.co.in
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
INTRODUCTION:
With the emergence of multidrug resistant Gram-negative bugs, there is an urgent need for rapid detection of these resistant organisms.
AIM:
This study was performed to assess the utility of a novel chromogenic medium in the detection of carbapenemase producing Enterobacteriaceae from various clinical specimens.
MATERIALS AND METHODS:
A total of 202 isolates of Enterobacteriaceae, which were resistant to meropenem (10 μg) disc by standard disc diffusion method, were analyzed over a period of 6 months. These isolates were subjected to the modified Hodge test (MHT) and inoculated onto the chromogenic medium. Following which the results were analyzed.
RESULTS:
It was observed that 76.73% of the Enterobacteriaceae gave a positive result on the MHT, 18.31% were negative and 4.95% gave indeterminate result. While 88.11% produced growth on the chromogenic medium, 6.93% yielded no growth and 4.95% gave variable results. Concordance in the results of the two tests was found to be 65.34%.
CONCLUSION:
Enterobacteriaceae are emerging to be carbapenem resistant and are responsible for the soaring rates of Healthcare Associated Infections (HAIs), especially among critically ill patients. Hence, early detection of the same is important in controlling HAIs.
Keywords
Carbapenemase producing Enterobacteriaceae
healthcare associated infection
modified Hodge test
Introduction
The postantibiotic era is witnessing the re-emergence of life threatening infections, especially in the form of Healthcare Associated Infections (HAIs). This has been majorly attributed to the increasing antimicrobial resistance, which is on the rise due to selective pressure of antibiotic usage, along with social and technical changes that enhance the transmission of microbes.[1] Enterobacteriaceae form a major part of the normal gut flora. Drug resistance in these Gram-negative bacilli (GNB) is responsible for rising rates of HAIs. These organisms are increasingly showing resistance to carbapenems, due to the production of the enzymes known as carbapenemases.[2] The genes for these enzymes are transferable via plasmids. The enzymes have been classified into Ambler; Class A of KPC type; Class B of the NDM-1, IMP, and VIM types; and Class D of OXA-48 type. They function by inactivating the carbapenems.[3]
Carbapenems, until now, were the “last line” drugs for the management of extended spectrum ß-lactamase producing Enterobacteriaceae.[4] Thus, as a crucial measure in hospital infection control and epidemiological investigations, it is important to identify Enterobacteriaceae that are resistant to these last line drugs.
Various phenotypic tests have been developed to screen for carbapenemase producing Enterobacteriaceae (CPE), one of which is the modified Hodge test (MHT). This is based on the detection of in vitro production of carbapenemases, mainly KPC and OXA-48 phenotypes.[5] The MHT is recommended by the Clinical and Laboratory Standards Institute (CLSI) for the confirmation of suspected carbapenemase production in Enterobacteriaceae.[6] Though time consuming, the MHT is easy to perform.
The HiCrome KPC agar is a commercially available agar-based medium that supports the growth of only CPE. The organisms can be identified based on the difference in the color of the colonies.[7] The data available regarding the utility of chromogenic media from India remains limited. This further reinforces the necessity for evaluating the utility and the need for using this media as an initial screening tool for the detection of CPE.
Molecular diagnosis remains the gold standard confirmatory test for the detection of carbapenemases. However, these tests are not readily available in resource limited settings, where the prevalence of CPE colonization, spread, and infection among hospitalized patients are on the rise.
In this study, we compare two approaches as methods for screening and detection of CPE; the MHT and a novel chromogenic medium-the HiCrome KPC agar.
Materials and Methods
Sampling, speciation and screening
The study was performed over a period of 6 months following the procurement of ethical clearance from the Institutional Ethics Committee.
Nonrepetitive isolates were obtained from various clinical samples sent for culture and sensitivity to the Microbiology Laboratory, M. S. Ramaiah Medical College and Hospitals, Bengaluru, India. The samples were processed according to the laboratory Standard Operating Procedures, i.e. inoculation on MacConkey's agar, cystine lactose electrolyte deficient agar, 5% sheep blood agar and chocolate agar. Based on the colony morphology and Gram reaction, the GNB were further speciated by biochemical reactions such as indole production, mannitol fermentation, motility, triple sugar iron agar, citrate utilization, and urea hydrolysis. The GNB were subjected to antibiotic susceptibility testing (AST) on Mueller Hinton agar (MHA) by Kirby Bauer disc diffusion method. The AST results were interpreted according to CLSI 2014 guidelines.
The Enterobacteriaceae that were nonsusceptible to meropenem (10 μg) disc (zone diameter ≤19 mm) were tested with the MHT and inoculated on the HiCrome KPC Agar.
The modified Hodge test
The MHT was performed according to CLSI 2014 guidelines. A 0.5 McFarland standard inoculum of Escherichia coli ATCC 25922 in 5 ml nutrient broth was prepared. It was further diluted with nutrient broth to obtain a 1:10 dilution of the original inoculum. This was inoculated as lawn culture on a 100 mm MHA plate and allowed to dry for 3–10 min. A 10 μg meropenem disc was placed in the center of the plate and using a sterile cotton swab, 3–5 isolated colonies of the test organism were picked and inoculated as a straight line outward from the edge of the disc to a length of 20–25 mm. For each of the tested isolates, Klebsiella pneumoniae ATCC BAA 1705 (blaKPC by PCR) and E. coli ATCC 25922 were used as positive and negative controls, respectively. After 16–20 h of incubation in ambient air at 37°C, the MHA plate was examined for growth around the controls and the test isolate at the intersection of the streak line and the zone of inhibition.[8] As shown in Figure 1, an enhanced growth in a “Clover-Leaf” shaped pattern at the intersection was suggestive of carbapenemase production, and any inhibition of the growth of E. coli ATCC 25922 lawn culture along the test organism streak line and this was regarded as an indeterminate result.

- The modified Hodge test: Clover-leaf shaped enhanced growth at the intersection
The chromogenic medium
HiCrome KPC Agar (M-1831, HiMedia Laboratories Pvt. Ltd., Mumbai, India) was prepared by suspending 16.5 g of dehydrated agar base in 500 ml of distilled water and sterilized by autoclaving at 15 lbs pressure at 121°C for 15 min. It was cooled to 45–50°C, and one vial of HiCrome KPC Selective Supplement (FD-279, HiMedia Laboratories Pvt. Ltd., Mumbai, India) was reconstituted in 5 ml of sterile distilled water added aseptically to the molten agar. The media was dispensed into 100 mm Petri plates and was allowed to dry. Media was also subjected to a sterility check. The performance of each batch of the HiCrome KPC agar was checked with K. pneumoniae ATCC BAA 1705 (blaKPC by PCR) and E. coli ATCC 25922 as positive and negative controls, respectively.
The test isolates were inoculated on the HiCrome KPC Agar and incubated in ambient air at 37°C for 18–24 h. The color produced by the colonies was compared with the manufacturer's instructions. E. coli and Citrobacter freundii produced pink to magenta colonies, while Klebsiella species, Enterobacter species, and Serratia species produced bluish-green colonies, and Salmonella species produced smooth colorless colonies [Figure 2].
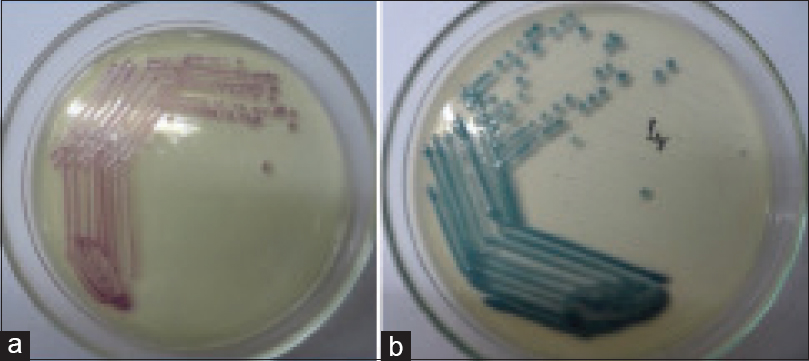
- Growth of carbapenem resistant Enterobacteriaceae on the HiCrome KPC Agar: (a) Pink to magenta colored colonies produced by Escherichia coli and Citrobacter freundii, (b) Bluish-green colored colonies produced by Klebsiella, Enterobacteri, and Serratia species
Analysis of results
Based on the literature survey, it was calculated that for a 95% confidence and 4% relative precision, the minimum of 162 samples was required to perform the study. The results were analyzed using IBM SPSS Statistics for Windows, Version 18.0 (IBM Corp. Armonk, NY, USA).
Results
A total of 202 isolates of Enterobacteriaceae that were nonsusceptible to meropenem by the disc-diffusion method, were obtained over a period of 6 months, of which 110 were Klebsiella species, 62 were E. coli, 16 belonged to Citrobacter species, 12 were Enterobacter aerogenes and two were Proteus species. The specimen wise distribution of the isolates is depicted in Table 1.
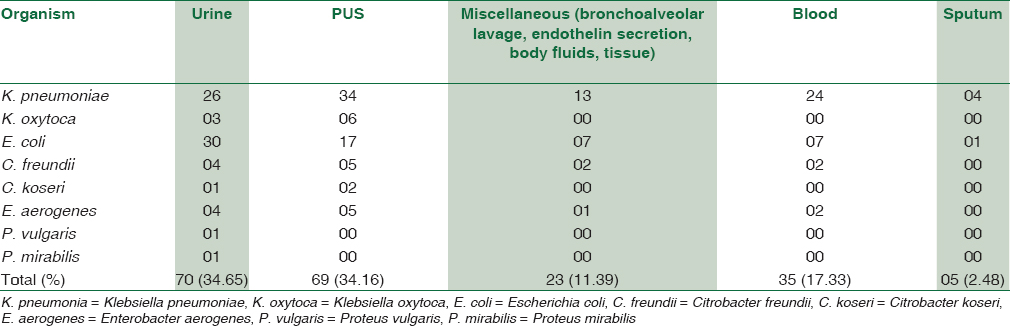
Out of the 202 isolates of Enterobacteriaceae, 155 (76.73%) formed a “Clover-Leaf” shaped indentation on the MHT, 37 (18.31%) were negative and 10 (4.95%) gave indeterminate results. On the HiCrome KPC agar, 178 (88.11%) isolates produced colored colonies that matched the manufacturer's instructions, 10 (4.95%) isolates grew with atypical color that could not be related to the manufacturers’ instructions while 14 (6.93%) of the Enterobacteriaceae did not produce any growth. The distribution of various Enterobacteriaceae based on the MHT and the growth on the HiCrome KPC Agar is depicted in Table 2.
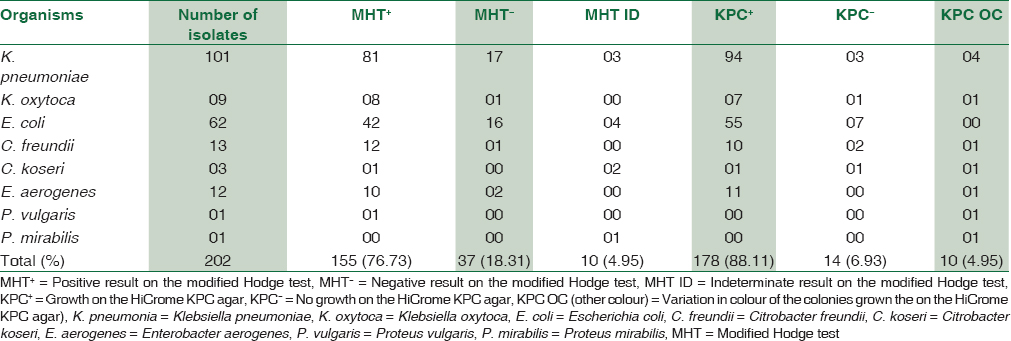
The results of the MHT and the HiCrome KPC Agar were consistent with each other in 132 (65.34%) of the isolates. The two methods yielded inconsistent results in a total of nine isolates that belonged to Klebsiella species, Citrobacter species and Enterobacter aerogenes. These isolates were positive on the MHT, but produced translucent white, bluish-green and pink-purple colonies on the HiCrome KPC Agar, respectively. It was observed that one isolate of Proteus mirabilis was MHT positive but produced translucent white colonies on the HiCrome KPC Agar. There was no discrepancy observed in any of the E. coli isolate, which showed 100% concordance in the results. Various combinations in the results of the MHT and HiCrome KPC Agar for different Enterobacteriaceae have been depicted in [Table 3].
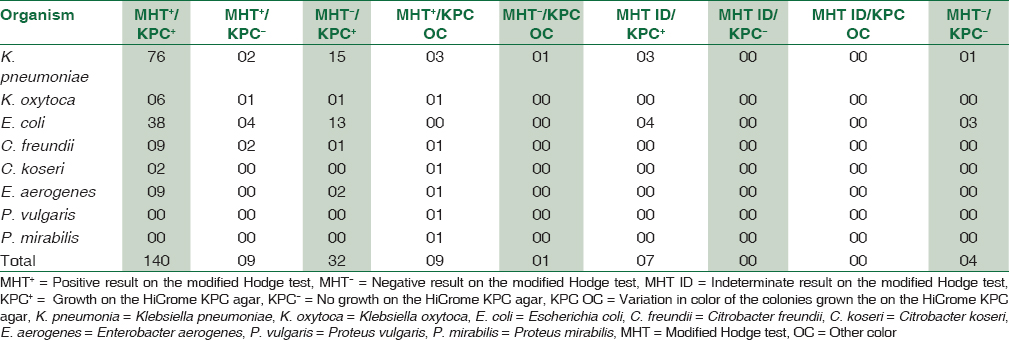
The cost of the HiCrome KPC Agar was calculated to be approximately the Indian National Rupee (INR) 315 per 100 mm Petri plate.
Discussion
Early isolation and detection of CPE can help control their spread in healthcare setting. Carbapenemase production among Enterobacteriaceae is an infection control emergency. Clinical microbiology laboratories play a critical role in the timely detection of the same, through investigation of surveillance samples and clinical samples. This can prove to be a crucial step in the appropriate management of patients while reinforcing infection prevention and control efforts.[910]
In the present study, the MHT could confirm 76.73% of the Enterobacteriaceae with reduced susceptibility to MP, while the HiCrome KPC Agar could detect 88.11%. 69.34% of isolates yielded consistent results with the two tests, while 20.29% yielded inconsistent results. The inconsistency could be attributed to the fact that the MHT detects KPC and OXA-48 phenotypes while the HiCrome KPC Agar is said to promote the growth of all carbapenemase producing phenotypes.
In a study conducted in Iran, the consistency in results between the MHT and KPC chromogenic medium were observed in 30 of the 244 (12.29%) isolates of K. pneumoniae that were screened based on MP disc diffusion method.[11]
Indeterminate results were observed in both the MHT and the HiCrome KPC Agar. According to CLSI, indeterminate results in the MHT do not have a clear microbiological or clinical interpretation. Such isolates require re-testing by molecular methods. Gilad et al. found that the strains that were KPC-nonproducing and carbepenem resistant, grew well on CHROMagar KPC, but with an atypical colony color.[1213] In this study, the production of carbepenemases other than KPC could be attributed to an atypical colony color on the HiCrome KPC Agar.
In another study, when used in rectal swab surveillance for CPE, the positive predictive value and negative predictive value of the KPC chromogenic medium was calculated as 100% and 98.8%, respectively.[7]
In a study by Gilad et al., the sensitivity of ChromAgar KPC was reported as 100% for KPC producing K. pneumoniae or E. cloacae and 71.4% for KPC producing E. coli.[13]
Rapid and timely, in vitro identification of CPE is of paramount importance in the prevention of its dissemination in healthcare setting, especially in the ICUs.[14]
This is one of the few studies from India where the MHT was compared with the HiCrome KPC Agar, and it was observed that the test results were not comparable. However, the HiCrome KPC Agar when used as an individual test can be a valuable screening method for rapid detection of CPE from clinical samples and surveillance cultures.[15] Moreover, the HiCrome KPC Agar has proven to be a cost and time effective screening method. The cost of the HiCrome KPC Agar was INR 315, while the cost of testing a single isolate was estimated to be approximately INR 176. The test results were obtained in the form of the genus level identification of the CPE. The turnaround time was 24 h for surveillance cultures. This methodology can play an integral role in the early adoption of hospital infection control measures in order to prevent the spread of CPE.
Limitations of the study
The sensitivity and the specificity of the tests with respect to the reference standard could not be assessed. Due to the limitation of resources, molecular detection for genes encoding for carbapenemase enzymes by PCR could not be performed.
Conclusion
It is essential to devise methods for the identification of CPE. These techniques should be cost and time effective as well as less labor intensive. The HiCrome KPC Agar is a novel and promising method that could supplement the MHT. This can help in direct screening of clinical isolates and surveillance cultures for CPE.
Though molecular methods remain the gold standard, these tests have not found a widespread use in resource limited countries.
The HiCrome KPC Agar can prove to be a credible method for prompt and timely detection of CPE in hospitals.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Antibiotic resistance mechanisms in bacteria: Biochemical and genetic aspects. Food Technol Biotechnol. 2008;46:11-21.
- [Google Scholar]
- Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect. 2012;18:432-8.
- [Google Scholar]
- Value of the modified Hodge test for detection of emerging carbapenemases in Enterobacteriaceae. J Clin Microbiol. 2012;50:477-9.
- [Google Scholar]
- Comparative evaluation of a chromogenic agar medium, the modified Hodge test, and a battery of meropenem-inhibitor discs for detection of carbapenemase activity in Enterobacteriaceae. J Clin Microbiol. 2011;49:1965-9.
- [Google Scholar]
- Evaluation of modified Hodge test as an indicator of Klebsiella pneumoniae carbapenemase (KPC) production by using bla kpc gene PCR. Int J Med Res Health Sci. 2014;3:65-70.
- [Google Scholar]
- Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791-8.
- [Google Scholar]
- Evaluation of CHROMagar™ KPC for the detection of carbapenemase-producing Enterobacteriaceae in rectal surveillance cultures. Int J Antimicrob Agents. 2011;37:124-8.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 24th Informational Supplement. CLSI Document M100-S24. Wayne, PA: Clinical Laboratory Standards Institute; 2014.
- Clinical microbiology costs for methods of active surveillance for Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Infect Control Hosp Epidemiol. 2014;35:350-5.
- [Google Scholar]
- Gram-negative bacteria that produce carbapenemases causing death attributed to recent foreign hospitalization. Antimicrob Agents Chemother. 2013;57:3085-91.
- [Google Scholar]
- A study on prevalence of KPC producing from Klebsiella pneumoniae using modified Hodge test and CHROMagar in Iran. Ann Biol Res. 2012;3:5659-64.
- [Google Scholar]
- The modified Hodge test is a useful tool for ruling out Klebsiella pneumoniae carbapenemase. Clinics (Sao Paulo). 2012;67:1427-31.
- [Google Scholar]
- Laboratory evaluation of the CHROMagar KPC medium for identification of carbapenem-nonsusceptible Enterobacteriaceae. Diagn Microbiol Infect Dis. 2011;70:565-7.
- [Google Scholar]
- Emergence of Klebsiella pneumoniae carbapenemase-producing bacteria. South Med J. 2011;104:40-5.
- [Google Scholar]
- Strategies for identification of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2013;68:487-9.
- [Google Scholar]





