Translate this page into:
Monocyte Distribution Width, a Novel Biomarker for Early Sepsis Screening and Comparison with Procalcitonin and C-Reactive Protein
Address for correspondence: Fatima Meraj, FCPS, Department of Hematology & Blood Center, Indus Hospital & Health Network, Korangi Campus, Plot C-76, Sector 31/5, Opposite Darussalam Society Korangi Crossing, Karachi, 75190, Pakistan (e-mail: fatima.meraj@tih.org.pk).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
Monocyte distribution width (MDW) can be used for the early recognition of sepsis. The study compared the diagnostic accuracy of the MDW with two well-known sepsis biomarkers, procalcitonin (PCT) and C-reactive protein (CRP).
Materials and Methods
A study was conducted from July 2021 to October 2021, on 111 patients admitted to the Indus Hospital and Health Network. Patients from the ages of 1 to 90 years were enrolled if hospitalized for more than 24 hours for suspected sepsis to avoid inclusion of patients who had short-term stay in the emergency department. According to the Sequential Organ Failure Assessment score, the clinical team did the characterization of cases as with sepsis or without sepsis. SPSS version 24 was used, and the diagnostic accuracy of MDW was assessed and compared using the area under the curves (AUCs) acquired from receiver operating characteristic curves. Pearson's chi-square/Fisher's exact test (as per need) was applied to determine the association. A p-value of less than 0.05 was considered significant.
Results
Among 111 patients, 81 (73%) patients were labeled with sepsis and 30 (27%) were without sepsis. We have reported significantly higher MDW, PCT, and CRP levels in septic patients (p < 0.001). The AUC of MDW was comparable with PCT (0.794). Significant cutoff value for the MDW was greater than 20.24 U with 86% sensitivity and 73% specificity.
Conclusion
MDW may have a predictive ability similar to PCT and CRP in terms of sepsis and, thus, can be used as a standard parameter for the timely diagnosis of sepsis.
Keywords
biomarkers
procalcitonin
C-reactive protein
monocyte distribution width
sepsis
Introduction
Sepsis has been reported as a major cause of increased morbidity, length of hospital stay, and mortality in health care systems worldwide.[1-6] The timely diagnosis and prompt management of sepsis are key components in controlling the progression of sepsis, as patients with less severe sepsis can progress to severe sepsis or septic shock within 72 hours if not intervened.[7] Its early detection is, however, a difficult step due to the absence of appropriate signs and symptoms.[8]
At present, the route to the detection of sepsis is based on two definitions, that is, Sepsis-2 and Sepsis-3. The criteria for Sepsis-2 was published in 1992 and defined as a documented infection in conjugation with a systemic inflammatory response syndrome score of 2 points[9] or higher whereas updated guidelines for sepsis management (SEPSIS-3) recommend the use of Sequential Organ Failure Assessment (SOFA) score, to recognize ensuing sepsis.[10] The complexities of the definition of sepsis, as well as the lack of a reliable biomarker, hamper early detection of sepsis in the emergency department (ED).
Several septic biomarkers proposed over the past 10 years such as interleukin-6 (IL-6), C-reactive protein (CRP), and procalcitonin (PCT), have been used for the early detection of sepsis.[11–13] However, sophisticated biomarkers are still restricted in real-world clinical practice. IL-6 is more specific for bacterial infection but it is not easily available.[14–16] PCT is not accessible in every first-level hospital, also it is not cost-effective, which makes it around four times more expensive than CRP.[16–18] Although CRP is commonly available, it has a lengthier turnaround time than a standard complete blood cell count (CBC) result which includes monocyte distribution width (MDW). These tests may be ordered by emergency physicians based on the findings of a standard CBC study conducted using a hematology analyzer. Most of the time, CBC results are reported earlier than clinical chemistry results.[19–21] If point-of-care CRP testing is not accessible, early antibiotic administration may be delayed.
The majority of modern automated hematology analyzers have upgraded cell counting functions that include nucleated red blood cells or immature granulocytes, which helps in the accurate quantification of peripheral blood cells in pathological conditions. In addition to a new quantity assessment, cell analysis technology may identify and provide some other parameters representing functional data of each leukocyte type which is also known as cell population data (CPD).[22,23]
Beckman Coulter Company introduced the UniCel DxH900, an advanced hematology analyzer, which has advanced CPD parameters that evaluate each leukocyte cell type. Early Sepsis Indicator (ESId) was proposed by Beckman Coulter Company and was further approved by the Food and Drug Administration in 2019 as the biomarker of sepsis.[24] In this instrument the function of the ESId is to assess the width of monocyte volumes (MDW). The UniCel DxH900 hematology analyzer reports this novel parameter with the CBC which claims to be helpful in the early detection of sepsis[25] because the neutrophils and monocytes play a vital role in the development of sepsis-induced inflammation and immunosuppression.[24–27] The goal of the study was to compare the diagnostic accuracy of the MDW for early detection of sepsis to two well-proven sepsis biomarkers, PCT and CRP.
Methodology
Patients
We conducted a cross-sectional observational study between July 2021 and October 2021, on 111 patients admitted to the Indus Hospital and Health Network. Patients from the ages of 1 to 90 years were consecutively enrolled if hospitalized for more than 24 hours for suspected sepsis to avoid inclusion of patients who has short-term stay in the ED. The clinical characterization of cases as sepsis versus without sepsis as per SOFA score was done by the clinical team. Past medical history and laboratory parameters (MDW, white blood cell count [WBC], PCT, and CRP) were collected from the Health Management Information System of the hospital. The patients with underlying chronic conditions (immunocompromised, malignancies, chronic kidney disease, or liver disease) were excluded as these comorbidities can facilitate organ dysfunction.
CBC Including MDW
In each patient, CBC was measured at the time of admission. Within the CBC, the parameters that were recorded included WBC (normal range: 4–10.5 × 109/L) and MDW. The optimal MDW cutoff value for sepsis detection greater than 20.0 U was taken from previously published literature.[28] Samples were collected in K2 ethylenediaminetetraacetic acid (EDTA) anticoagulant tubes, and CBC was determined by UniCel DxH 900 hematology analyzer by using advanced volume, conductivity, and scatter 360 and data fusion technology. Concisely, scatter signals can evaluate morphological changes in leukocytes, particularly in monocytes. Hence, the value of MDW signifies a measure of the dispersion around the mean of the monocyte volume population. Following manufacturer directions, maintenance, functions, and calibration were performed. For the assessment of quality control, the Coulter 6C Cell Controls were used with three different concentration levels. The analysis was performed by using the 1.1.0 software version.
PCT
For the determination of PCT, Cobas e 411 analyzer (F. Hoffmann-La Roche Ltd.) was used. It is an automated analyzer using electrochemiluminescence technology for immunoassay analysis. The cutoff value for PCT was 0.05 ng/mL. Our reference ranges for PCT values were as per the previously published literature by Meisner[29] (i.e., < 0.05 healthy adult, 0.05 to < 0.5 systemic infection is unlikely although localized infection is possible, 0.5 to < 2 systemic infections are possible but other conditions [e.g., major trauma, recent surgery, severe cardiogenic shock] may also induce significant PCT rises, 2 to < 10 systemic infections is likely, and ≥ 10 high likelihood of severe bacterial sepsis or septic shock).[29]
CRP
Serum CRP was evaluated by using a photometric/potentiometric technique of Alinity c (Abbot, Abbott Park, Illinois, United States), according to the manufacturer's instructions. The cutoff value for positive CRP is greater than 05 mg/L.[30]
Statistical Analysis
SPSS version 24 was used to perform statistical analysis. Median and interquartile ranges (IQRs) were calculated for quantitative variables like age, PCT, CRP, MDW, and WBC; and qualitative variable like gender was presented as percentages. The diagnostic accuracy of MDW was assessed and compared using the area under the curves (AUCs) acquired from receiver operating characteristic curves. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio, and negative likelihood ratio were also calculated. Pearson's chi-square/Fisher's exact test (as per need) was applied to determine the association between different categories of PCT with MDW and for the association of different markers like PCT, MDW, and CRP in patients with or without sepsis. A p-value of less than 0.05 was considered significant.
Results
During the study period, a total of 111 patients were enrolled of which males were 55 (49.5%) and females were 56 (50.5%). There were 98 (88.3%) adults and 13 (11.7%) children. The median (range) (IQR) of pediatric and adult patients' ages were 3 (1–17) (2–13.50) and 55.50 (18–90) (38.75–64) years, respectively.
Among the 111 enrolled patients, 81 (73%) patients were labeled with sepsis and 30 (27%) were defined as having no sepsis, as retrieved from the medical record. Demographics and biochemical data of the two groups investigated are shown in ►Table 1. No differences in age and WBC were found between the groups. We have reported significantly higher MDW, PCT, and CRP levels in septic patients (p < 0.001 for all comparisons) (►Table 1).
| Variables | Sepsis (81) | No sepsis (30) | p-Value |
|---|---|---|---|
| Demographics | |||
| Gender (N) (%) | 0.012ac | ||
| Male | 46 (56.8) | 09 (30) | |
| Female | 35 (43.2) | 21 (70) | |
| Age (median) (IQR) | 50 (24.75–62.75) | 51 (37.50–61.75) | 0.811b |
| Laboratory parameters (median) (IQR) | |||
| MDW, U | 25.5 (22.8–29.4) | 18.7 (17.3–21.3) | 0.000bc |
| PCT, ng/mL | 0.23 (0.11–0.95) | 0.039 (0.033–0.094) | 0.000bc |
| CRP, mg/L | 65.7 (31.1–140.4) | 10.1 (4.8–31.2) | 0.000bc |
| WBC × 103/μL | 11.4 (8.2–15.3) | 9 (6.3–13.5) | 0.118b |
| Outcome (N) (%) | |||
| Mortality | 19 (23.5) | 0 | 0.004ac |
Abbreviations: CRP, C-reactive protein; IQR, interquartile range; MDW, monocyte distribution width; PCT, procalcitonin; WBC, white blood cell count.
a Pearson's chi-square.
b Mann–Whitney U.
c Significant value.
The present study reported the median (IQR) of PCT as 0.174 ng/mL (0.0740–0.995) and MDW as 24.90 U (20.3–32.08), respectively. There were 92 (82.9%) patients who were positive for PCT, that is, 0.05 ng/mL or more, while negative PCT values (< 0.05 ng/mL) were present in 19 (17.1%) patients. We compared the diagnostic performance in sepsis prediction, the AUC of MDW was found to be comparable with that of PCT (0.794, 95% confidence interval [CI]: 0.652–0.936; p-value 0.000). At 20.24 U or more, the maximum sensitivity of MDW with PCT was 84.8%, specificity was 73.68%, PPV was 94.12%, and NPV was 53.85% (►Fig. 1A).

- (A–D) Receiver operating characteristic (ROC) curves comparison of (A) monocyte distribution width (MDW)-procalcitonin (PCT), (B) MDW-C-reactive protein (CRP), (C) MDW-white blood cell count (WBC), and (D) MDW-sepsis. Area under the curve (AUC) estimates along with their 95% confidence interval are shown in the “Results” section.
Total CRP was done in 77 patients, the median (IQR) of CRP was 45.80 mg/L (13.65–119.80) of which negative were 11 (14.3%) while positive were 66 (85.7%). We compared the diagnostic performance in sepsis prediction, the AUC of MDW with CRP was 0.777, 95% CI: 0.491–1.000; p-value 0.026. At 20.24 U or more, the maximum sensitivity of MDW with PCT was 77.3%, specificity was 83.3%, PPV was 80.30%, and NPV was 45.45% (►Fig. 1B).
The median (IQR) of WBC was 11.30 × 109/L (7.2–15.3); 45 (40.5%) were in normal ranges while 66 (59.5%) were in abnormal ranges. We compared the diagnostic performance in sepsis prediction, the AUC of MDW with WBC, that is, 0.604, was very poor with the 95% CI of 0.496–0.712 having a nonsignificant p-value. At 20.24 U or higher cutoff, the maximum sensitivity of MDW was 63.53%, specificity was 53.85%, PPV was 81.82%, and NPV was 31.11% as shown in ►Fig. 1C.
Out of the 111patient, sepsis was present in 81 (73%) and no sepsis in 30 (27%) patients. At 20.24 U or higher cutoff, the maximum sensitivity of MDW was 93.8%, specificity was 70%, PPV was 89.4%, and NPV was 80.7% (AUC 0.865; CI: 0.777–0.953) as shown in ►Fig. 1D.
We have observed a significant positive Spearman's correlation of MDW and PCT which is 0.382, p-value less than 0.001, and nonsignificant positive Spearman's correlation with CRP which is 0.223, and a nonsignificant negative Spearman's correlation with WBC which is –0.139.
The findings of MDW as per clinical characterization of PCT, that is, less than 0.05 healthy adult, 0.05 to less than 0.5 systemic infection is unlikely although localized infection is possible, 0.5 to less than 2 systemic infections are possible but other conditions may exist, 2 to less than 10 systemic infection is likely, and 10 or higher likelihood of severe bacterial sepsis or septic shock, are shown in ►Table 2.
| PCT (N = 111) ng/mL | < 0.05 (n = 19) | 0.05–< 0.5 (n = 56) | 0.5–< 2 (n = 16) | 2–< 10 (n = 9) | ≥ 10 (n = 11) |
|---|---|---|---|---|---|
| MDW | |||||
| < 20 (n) (%) | 14 (73.7) | 9 (16.1) | 1 (6.3) | 2 (22.2) | 0 |
| > 20 (n) (%) | 5 (26.3) | 47 (83.9) | 15 (93.7) | 7 (77.8) | 11 (100) |
| p-Value | 0.000ab | 0.000ab | 0.000ab | 0.017ab | 0.000ab |
| AUC (95% CI) | 0.794 (0.65–0.936) | 0.773 (0.625–0.922) | 0.836 (0.691–0.980) | 0.772 (0.598–0.946) | 0.856 (0.715–0.998) |
| Sensitivity (%) (95% CI) | 53.85 (33.7–73.41) | 83.93 (71.67–92.38) | 93.75 (66.77–99.84) | 77.78 (39.99–97.19) | 100 (71.51–100) |
| Specificity (%) (95% CI) | 94.12 (86.8–98.06) | 73.68 (48.80–90.85) | 73.68 (48.80–90.85) | 73.68 (48.80–90.85) | 73.68 (48.80–90.85) |
| LR+ (ratio) | 9.15 | 3.19 | 3.56 | 2.96 | 3.80 |
| LR– (ratio) | 0.49 | 0.22 | 0.08 | 0.30 | 0.00 |
| PPV (%) | 73.68 | 90.38 | 75.00 | 58.33 | 68.75 |
| NPV (%) | 86.96 | 60.87 | 93.33 | 87.50 | 100 |
Abbreviations: AUC, area under the curve; CI, confidence interval; LR, likelihood ratio; MDW, monocyte distribution width; NPV, negative predictive value; PCT, procalcitonin; PPV, positive predictive value.
a Fisher's exact test.
b Significant value.
In the negative category of PCT (≤ 0.05 ng/mL; ►Table 2), 05 cases showed raised MDW (> 20 U) irrespective of PCT. On close follow-up of these cases, 3 were diagnosed with coronavirus disease 2019, who later developed signs and symptoms of sepsis but PCT was negative which was tested one time, this favors that MDW can detect early sepsis when PCT is normal. However, the sample size of this study is small. To prove this a bigger sample size study should be done. The rest of the two cases were not followed properly as one patient left against medical advice and the other was diagnosed as urethral stricture.
We have also determined the AUC of MDW 0.874 (95% CI: 0.772–0.9716; p < 0.000), PCT 0.861 (95% CI: 0.766–0.956; p < 0.001), CRP 0.796 (95% CI: 0.679–0.912; p = 0.000), and WBC 0.599 (95% CI: 0.452–0.746) in patients with sepsis (73%) and without sepsis (27%), as displayed in ►Fig. 2.
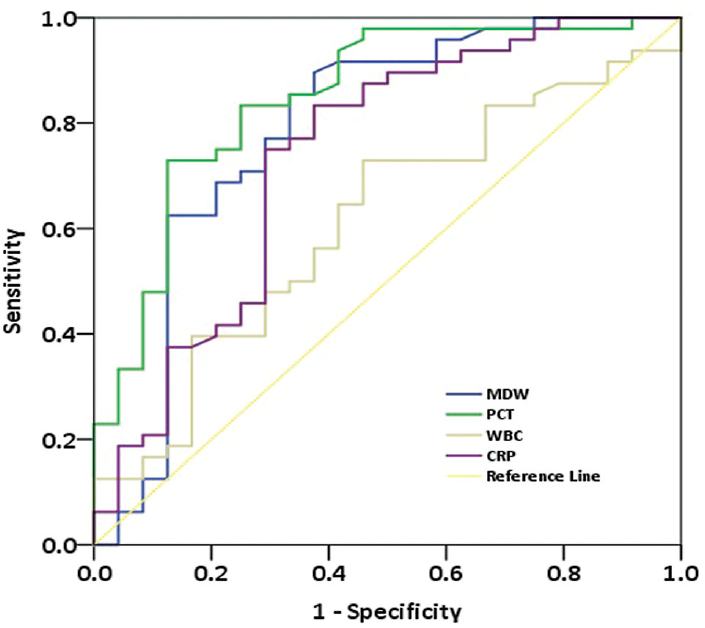
- Receiver operating characteristic (ROC) curve of monocyte distribution width (MDW), procalcitonin (PCT), C-reactive protein (CRP), and white blood cell count (WBC) in patients with or without sepsis.
Discussion
In this study, we evaluated the diagnostic accuracy of MDW which is the measure of morphologic heterogeneity resulting from an immune response to bacterial infection, for early sepsis detection in the ED. The main findings of our study are as follows: (1) Significantly higher MDW, PCT, and CRP were reported in patients with sepsis compared with those without sepsis. (2) The best cutoff value of MDW was 20.24 or greater for detecting sepsis. (3) We have observed good AUC of MDW 0.874 and PCT 0.861 in sepsis patients, fair AUC with CRP 0.796, and poor WBC with 0.604. (4) We have calculated the AUC for each PCT category with MDW, that is, good AUC 0.836 and 0.856 in 0.5 to less than 2 and 10 ng/mL or greater, respectively, while fair AUC 0.794, 0.773, and 0.772 in less than 0.05, 0.05 to less than 0.5, and 2 to less than 10 ng/mL, respectively.
The preliminary evidence presented by relevant investigations was consistent with our findings. Recent research suggests that variations in monocyte volume may be attributable to monocyte activation as part of the innate immune response to bloodstream infections.[31-33]
In our study, the cutoff value of MDW was 20.24 U, which is similar to 20.0 U[28] and 20.5 U[31,34] observed in other studies, while Polilli et al and Piva et al reported 21.9 U[32] and 24.63 U[35] MDW cutoff values, respectively. Agnello et al reported 23.5 U as the cutoff MDW value for sepsis detection.[36]
Crouser et al assessed the diagnostic performance of MDW, they infer that MDW had the best discriminator power for sepsis, based on either Sepsis-2[28,31] or Sepsis-3[28] criteria, with an AUC of 0.79, with the sensitivity and specificity of 77 and 73%, respectively, at a cutoff point of 20.50 U. Also, they discovered that combining MDW with WBC increased sepsis detection when compared with WBC or MDW alone. But they did not compare MDW with other sepsis indicators like CRP.
A study by Agnello et al compared MDW to CRP, which is similar to our study. CRP is the most often used inflammatory biomarker in the ED and all clinical settings. MDW, as part of the CBC, has the benefit of being available to physicians very early during clinical assessment, when the sepsis diagnosis could not be considered[36]; CRP, along with PCT, is necessary when doctors suspect sepsis. They found that MDW at 23.5 U had a NPV of 99.6%, indicating that a value below this cutoff allows for excellent accuracy in eliminating sepsis in a typical ED group. These findings add to MDW's value to practitioners as a clinical evaluation tool for ruling out sepsis and avoiding unneeded and expensive therapies.[37]
The cutoff value of our study was similar to Crouser et al[28,31] while different from Agnello et al.[36] This difference can be explained by the influence of anticoagulant (K2-EDTA vs. K3-EDTA) used for blood collection. The effect of anticoagulant on MDW has been described by the manufacturer in the manual (https://www.beckmancoulter.com/download/file/wsr-262828/C21894AC?type=pdf). Crouser et al performed their study on the sample collected in K2-EDTA same as our study, while Agnello et al[38] measured MDW in K3-EDTA tubes. Thus, it is recommended by the manufacturer not to use the same cutoff MDW value for K2- or K3-EDTA and rather establish its cutoff value which will integrate not only the type of anticoagulant but also the demographical characteristics of the population.
Another study by Polilli et al also examined the diagnostic performance of MDW and PCT, the authors concluded that MDW was as good as PCT at predicting sepsis, finding that their AUCs were substantially identical.[32] Even though PCT is still the most widely validated biomarker for sepsis,[38] its usage in the ED has numerous drawbacks, including availability and cost-effectiveness.[39]
The study has limitations; first, this is a single-center study with a limited sample size. Second, we were not able to correlate MDW with the blood cultures and lactate levels, as they were not requested in most patients. The last limitation was the serial estimation of all the septic markers including MDW was not being followed along with the course of the disease. A study with large sample size is required for addressing the limitations of this study.
In lower to middle-income countries like Pakistan, we have very limited resources for diagnosis and treatment. If we can predict sepsis on CBC by observing MDW it will cut down our cost as it is a routine parameter of CBC and in turn, this will illicit early management of patients thus improving their outcome.
Conclusion
In this study, we compared clinically septic patients with laboratory parameters and a novel septic marker, that is, MDW. We found promising results of MDW for sepsis prediction. MDW can be measured automatically within a few minutes without additional cost through routine CBC. This could promptly identify patients at high risk of sepsis, whose diagnosis can be confirmed by further clinical and other laboratory markers depending on the availability of the test, therefore preventing life-threatening septic shock. Further studies are needed to validate and establish its utility as an early septic marker.
Authors' Contributions
F.M. and S.S. conceived the idea and designed the study. S.M. analyzed, interpreted the patient data, and did the initial drafting of the manuscript. F.K., H.K., and S.J. reviewed critically for important intellectual content. All authors read and approved the final manuscript.
Note
IRB exemption was given before the start of the study (IRB Number: IRD_IRB_2021_06_002).
Conflict of Interest
None declared.
Funding
None.
References
- The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546-1554.
- [CrossRef] [PubMed] [Google Scholar]
- ARISE Investigators, for the Australian and New Zealand Intensive Care Society Clinical Trials Group. Australasian resuscitation of sepsis evaluation (ARISE): a multi-centre, prospective, inception cohort study. Resuscitation. 2009;80(07):811-818.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(05):1244-1250.
- [CrossRef] [PubMed] [Google Scholar]
- ICON investigators. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2(05):380-386.
- [CrossRef] [PubMed] [Google Scholar]
- International Forum of Acute Care Trialists. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(03):259-272.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of sepsis and its treatment measures on intestinal flora structure in critical care patients. World J Gastroenterol. 2021;27(19):2376-2393.
- [CrossRef] [PubMed] [Google Scholar]
- Disease progression in hemodynamically stable patients presenting to the emergency department with sepsis. Acad Emerg Med. 2010;17(04):383-390.
- [CrossRef] [PubMed] [Google Scholar]
- The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(06):1644-1655.
- [CrossRef] [PubMed] [Google Scholar]
- The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(08):801-810.
- [CrossRef] [PubMed] [Google Scholar]
- Monocyte distribution width as a biomarker of sepsis in the intensive care unit: a pilot study. Ann Clin Biochem. 2021;58(01):70-73.
- [CrossRef] [PubMed] [Google Scholar]
- Improved diagnostic and prognostic power of combined delta neutrophil index and mean platelet volume in pediatric sepsis. Ann Clin Lab Sci. 2018;48(02):223-230.
- [Google Scholar]
- Diagnostic accuracy of procalcitonin, neutrophil-lymphocyte count ratio, C-reactive protein, and lactate in patients with suspected bacterial sepsis. PLoS One. 2017;12(07):e0181704.
- [CrossRef] [PubMed] [Google Scholar]
- Use of procalcitonin for the prediction and treatment of acute bacterial infection in children. Curr Opin Pediatr. 2014;26(03):292-298.
- [CrossRef] [PubMed] [Google Scholar]
- Procalcitonin for guidance of antibiotic therapy. Expert Rev Anti Infect Ther. 2010;8(05):575-587.
- [CrossRef] [PubMed] [Google Scholar]
- Procalcitonin and C-reactive protein as markers of systemic inflammatory response syndrome severity in critically ill children. Intensive Care Med. 2007;33(03):477-484.
- [CrossRef] [PubMed] [Google Scholar]
- Procalcitonin biomarker kinetics to predict multiorgan dysfunction syndrome in children with sepsis and systemic inflammatory response syndrome. Iran J Pediatr. 2015;25(01):e324.
- [CrossRef] [PubMed] [Google Scholar]
- Procalcitonin does discriminate between sepsis and systemic inflammatory response syndrome. Arch Dis Child. 2006;91(02):117-120.
- [CrossRef] [PubMed] [Google Scholar]
- A point-of-care chemistry test for reduction of turnaround and clinical decision time. Am J Emerg Med. 2011;29(05):489-495.
- [CrossRef] [PubMed] [Google Scholar]
- Use of point-of-care testing and early assessment model reduces length of stay for ambulatory patients in an emergency department. Scand J Trauma Resusc Emerg Med. 2016;24(01):125.
- [CrossRef] [PubMed] [Google Scholar]
- Role of leucocytes cell population data in the early detection of sepsis. J Clin Pathol. 2018;71(03):259-266.
- [CrossRef] [PubMed] [Google Scholar]
- Improvement in detecting sepsis using leukocyte cell population data (CPD) Clin Chem Lab Med. 2019;57(06):918-926.
- [CrossRef] [PubMed] [Google Scholar]
- Coulter's Early Sepsis Indicator Receives 510(k) Clearance from the U.S. Food and Drug Administration [Internet] Beckmancoulter.com. Accessed October 25, 2021, at: https://www.beckmancoulter.com/about-beckman-coulter/newsroom/press-releases/2019/q2/2019-18-april-bec-esid-fda-clearance
- [Google Scholar]
- Automated determination of neutrophil volume as screening test for late-onset sepsis in very low birth infants. Pediatr Infect Dis J. 2010;29(03):288.
- [CrossRef] [PubMed] [Google Scholar]
- Automated determination of neutrophil VCS parameters in diagnosis and treatment efficacy of neonatal sepsis. Pediatr Res. 2012;71(01):121-125.
- [CrossRef] [PubMed] [Google Scholar]
- Volume, conductivity and scatter properties of leukocytes (VCS technology) in detecting sepsis in critically ill adult patients. Blood. 2011;118(21):4729.
- [CrossRef] [Google Scholar]
- Monocyte distribution width: a novel indicator of Sepsis-2 and Sepsis-3 in high-risk emergency department patients. Crit Care Med. 2019;47(08):1018-1025.
- [CrossRef] [PubMed] [Google Scholar]
- Procalcitonin: Biochemistry and Clinical Diagnosis. Dresden, Germany: UNI-MED-Verlag; 2010.
- [Google Scholar]
- 2010 Accessed June 4, 2022, at: https://www.ilexmedical.com/files/PDF/CRPVARIO_ARC_CHEM.pdf
- Improved early detection of sepsis in the ED with a novel monocyte distribution width biomarker. Chest. 2017;152(03):518-526.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of monocyte distribution width (MDW) and procalcitonin for early recognition of sepsis. PLoS One. 2020;15(01):e0227300.
- [CrossRef] [PubMed] [Google Scholar]
- Reviewing the value of leukocytes cell population data (CPD) in the management of sepsis. Ann Transl Med. 2020;8(15):953.
- [CrossRef] [PubMed] [Google Scholar]
- Monocyte distribution width compared with C-reactive protein and procalcitonin for early sepsis detection in the emergency department. PLoS One. 2021;16(04):e0250101.
- [CrossRef] [PubMed] [Google Scholar]
- Monocyte distribution width (MDW) parameter as a sepsis indicator in intensive care units. Clin Chem Lab Med. 2021;59(07):1307-1314.
- [CrossRef] [PubMed] [Google Scholar]
- Monocyte distribution width (MDW) as a screening tool for sepsis in the emergency department. Clin Chem Lab Med. 2020;58(11):1951-1957.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding negative predictive value of diagnostic tests used in clinical practice. Dimens Crit Care Nurs. 2017;36(01):22-29.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of serum procalcitonin and C-reactive protein in severity assessment of community-acquired pneumonia in children. Clin Biochem. 2016;49(1-2):47-50.
- [CrossRef] [PubMed] [Google Scholar]
- The leukocyte VCS parameters compared with procalcitonin, interleukin-6, and soluble hemoglobin scavenger receptor sCD163 for prediction of sepsis in patients with cirrhosis. Dis Markers. 2019;2019 1369798
- [CrossRef] [PubMed] [Google Scholar]






