Translate this page into:
VG111: A novel formulation demonstrating clinical evidence of anti-pathogenic activity and accelerated wound healing in humans and companion animals
*Corresponding author: Vikas Gautam, Department of Medical Microbiology, Post Graduate Institute of Medical Education and Research, Chandigarh, India. r_vg@yahoo.co.uk
-
Received: ,
Accepted: ,
How to cite this article: Singhal L, Singh KA, Panda RK, Malik YS, Verma RK, Ghosh D, et al. VG111: A novel formulation demonstrating clinical evidence of anti-pathogenic activity and accelerated wound healing in humans and companion animals. J Lab Physicians. 2024;16:347-57. doi: 10.25259/JLP_29_2024
Abstract
Objectives:
The increasing incidence of chronic wounds such as diabetic foot ulcers and pressure ulcers, often compounded by bacterial infections and biofilm formation, presents significant challenges in wound management. Despite advancements in wound care products and a better understanding of molecular wound repair mechanisms, the treatment of chronic ulcerating conditions remains incomplete. VG111, a novel natural product formulation, emerges as a promising therapeutic candidate addressing the need for an effective wound healing agent with antimicrobial and tissue regenerative properties.
Materials and Methods:
A thorough evaluation of VG111 included antimicrobial assays to determine its minimum inhibitory concentration against an array of pathogens, assessment of its biofilm disruption capabilities, investigation into its profibrogenic activity through scratch assays, and analysis of its immunomodulatory effects on macrophage-derived cytokines. Quality consistency was ensured by high-performance liquid chromatography fingerprinting, while clinical applicability was assessed through observations in canine and human wound healing cases.
Statistical Analysis:
The cytotoxic effects of VG111 were assessed using a Two-way ANOVA, indicating no significant cytotoxicity at the tested concentration (Column factor p<0.0001).
Results:
VG111 demonstrated potent antimicrobial action with effective concentrations ranging from 2.5% to 5.0% v/v, targeting resistant strains of Methicillin-resistant Staphylococcus aureus, colistin-resistant Escherichia coli, Acinetobacter baumannii, and other priority pathogens. It showed biofilm clearance, enhanced fibroblast migration, and a favorable immunomodulatory profile by reducing inflammatory cytokines in vitro. In vivo applications corroborated these findings, with significant wound healing observed in both veterinary and clinical settings, negating the need for additional antibiotics.
Conclusions:
The study emphasized on VG111 as a robust wound healing agent with significant antimicrobial and biofilm-disrupting properties. Its broad-spectrum efficacy against critical pathogens and ability to promote tissue regeneration mark it as a promising avenue in the management of complex chronic wounds, meriting further clinical exploration.
Keywords
Wound healing
Chronic wounds
Diabetic ulcers
Antimicrobial
Anti-biofilm
INTRODUCTION
Wound care, an ancient practice with historical roots, has historically grappled with complications stemming from both wound-related cutaneous issues and systemic challenges. However, the past two decades have witnessed remarkable advancements in wound management, fostering the creation of contemporary wound care products such as absorbent dressings constructed from alginates, chitosan, and various natural or synthetic drug-based materials.[1] Concurrently, there has been a growing emphasis on revitalizing the molecular aspects of wound repair. In pursuit of this goal, topical applications grounded in growth factors, honey, essential oils, and other natural agents have garnered attention due to their proven biosafety and documented efficacy in promoting tissue regeneration.[2-5] However, their applications in chronic ulcerating conditions remain incompletely explored.
Wounds can be classified fundamentally into acute and chronic categories based on their severity and clinical attributes. Acute wounds are expected to heal rapidly, while chronic wounds typically stem from long-standing injuries or ulcers. Chronic cutaneous wounds are defined by a healing period of three months or more and are vulnerable to opportunistic pathogens and adverse circumstances, encompassing chronic pressure ulcers (PUs) (PUs/bedsores) and diabetic foot ulcers (DFUs).[6] These conditions frequently manifest in individuals who already grapple with metabolic disorders such as diabetes or conditions leading to compromised blood supply in the vicinity of the wound.[7] Notably, DFUs and PUs exhibit global prevalence rates of 3–13% and 8.8–53.2%, respectively, with diabetes carrying an elevated risk of lower limb amputation and exacerbation of bedsores in surgical patients.[8-11] Shockingly, a lower limb is amputated worldwide every 30s, with India bearing the highest proportion of diabetes-afflicted individuals and the highest rate of diabetes-related foot disorders, a fifth of which necessitate amputation.[12-15] In addition, DFUs significantly impact an individual’s social and psychological well-being, resulting in reduced social interactions, restricted career prospects, and financial hardships.[16]
Chronic wounds also exhibit heightened susceptibility to nosocomial infections and opportunistic pathogens. Diabetic foot disease and PUs often emerge from a trio of factors: ischemia, neuropathy, and infection.[17,18] If untreated, these infections can extend to deeper tissues, typically in a contiguous manner.[19] The predominant chronic wound colonization and infections are attributed to the presence of normal skin microbiota such as Staphylococcus epidermidis, other coagulase-negative Staphylococcus spp., Staphylococcus aureus, and Streptococcus pyogenes, Gram-negative bacteria including Escherichia coli, Klebsiella, Pseudomonas, Acinetobacter, and Stenotrophomonas.[7,20] The colonization of wound surfaces by potential pathogens is a primary contributing factor to the worsening of chronic wounds in clinical environments.[20] Moreover, delayed wound healing fosters the formation of recalcitrant microbial biofilms, further complicating wound management. These biofilms not only confer antimicrobial resistance to the resident flora but also provide a shield against the host’s inflammatory response.[21] Cases of DFUs and PUs/bedsores serve as prominent examples of this complex scenario observed in clinical settings.
Despite the ongoing strides in wound care therapies, the quest for an optimal solution that substantially ameliorates ulcerative conditions remains unfulfilled.[22] To address these concerns, a novel natural product formulation was developed at the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh (patent no. 353978, accessible through https://iprsearch.ipindia.gov.in/publicsearch). This study focuses on VG111 for its antimicrobial activity, wound healing efficacy, and tissue regenerative properties. The recently published study underscores VG111’s potential to overcome the limitations of existing wound care products, offering rapid healing in 13 clinical cases and also showcasing its broad-spectrum antimicrobial activity, including against multidrug-resistant bacteria.[23] The results obtained from the present study suggest that VG111 might not only improve clinical outcomes but also enhance the quality of life for those who bear the burden of chronic wounds by focusing on the mechanistic insights of VG111 in diverse experimental settings.
MATERIALS AND METHODS
VG111 is an oil-based formulation that was used in two different forms: (i) the formulation as such, referred to as VG111, and (ii) the aqueous extract of ingredients without oil, referred to as AE-VG111 here on.
Antibacterial activity: Broth microdilution testing (PGIMER, Chandigarh)
AE-VG111 was subsequently diluted and treated against selected identified pathogens to elucidate the MIC breakpoints. Broth microdilution was performed in a 96-well U-bottom microtiter plate. The final volume of solution in each well was 100 μL (50 μL Mueller-Hinton broth 2× [MHB] + 25 μL bacterial suspension [10 μL 0.5 McFarland bacterial suspension diluted in 740 μL normal saline] + 25 μL of AE-VG111 in varying concentrations). Tetrazolium chloride (1%, 10 μL) was added after 18 h incubation at 37°C and then re-incubated for 30 min for pigment-based biomass detection.
Determination of reactive oxygen species (ROS) generation in bacterial specimens by VG111 (Institute of Nano Science and Technology [INST], Mohali)
For determining ROS generation in the treated bacterial specimens, 2,7-dichlorofluorescein diacetate (DCFDA) dye was used. Briefly, the bacteria were treated with AE-VG111, and post-treatment, the bacterial pellet was collected through centrifugation. DCFDA dye at a final concentration of 50 μg/mL was added and incubated for 30 min in the dark. Hydrogen peroxide (200 μM) was used as a positive control. The ROS generated in the supernatant was determined at 488 nm excitation and 530 nm emission using a Tecan plate reader.[24]
Bacterial biofilm clearance under scanning electron microscope (SEM) (PGIMER, Chandigarh)
For biofilm surface preparation, on day one, 2.5 mL of 1:100 dilution of 0.5 McF Pseudomonas aeruginosa PA14 (in tryptic soy broth (TSB)) and Stenotrophomonas sepilia sp. nov. was added on a cover slip installed into A1 and B1 well, respectively, followed by incubation at 37°C for 24 h. Media were removed from A1 and B1 wells and washed twice with distilled water on day 2. This was followed by the addition of AE-VG111 in well A1. Another coverslip was added to respective 2nd Wells A2 and B2 where again 2.5 mL of 1:100 dilution of 0.5 McFarland PA14 and S. sepilia (in TSB) was added and then incubated at 37°C for 24 h. After media removal and washing on 3rd day, wells A1, A2, A3, and A4 were fixated with 2.5% glutaraldehyde before overnight incubation at 4°C. Fixative was removed on day 4 with 0.1 M Sorenson’s buffer, followed by dehydration for 20 min. The dehydrated biofilm surfaces were then submitted for SEM sample preparation and visualization in a Central sophisticated instrumentation cell, PGIMER, Chandigarh (JSM-IT300 JEOL Ltd. Japan).
Fibroblast migration assay, scratch test (INST, Mohali)
A confluent monolayer of fibroblast cells was obtained by seeding ~2 × 106 cells per well in 6-well plates and growing them for 48 h. A scratch wound (∼400 μm) was made in each well by streaking a sterile 200 μL pipette tip across the monolayer. The culture medium was replaced with serum-free medium for 24 h, then complete medium (supplemented with 5% fetal bovine serum (FBS)) with or without AE-VG111 (equivalent concentration of 2% [v/v]) was added while the wound was allowed to heal for 48 h. The percentage of the scratch wound area that was freshly covered with fibroblasts was measured to evaluate migration. The magnitude of wound closure was estimated by computing the width and area of the wound at 0, 6, 12, and 24 h after treatment.
Cytokine and matrix metalloproteinase (MMP) estimation (INST, Mohali)
Levels of pro- and anti-inflammatory cytokines, in addition to MMPs, were estimated as indicators of inflammation. The effect of AE-VG111 on these markers was observed on treating RAW464.7 mouse macrophages and determining the levels with an enzyme-linked immunosorbent assay (Krishgen Biosystems, Mumbai, India) according to the manufacturer’s instructions. For details of the method used, refer to the supplemental method sections SM1 and SM2.
Cytotoxicity determination of VG111
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (PGIMER, Chandigarh)
Human keratinocyte cell lines were used for the MTT assay. To achieve this, cells were grown in a 96-well microtiter plate in Dulbecco’s modified eagle medium (DMEM) and cell confluency of 70–80% cells were treated with 100% (v/v) AE-VG111. The plate was incubated for 24 h at 37°C with 5% carbon dioxide (CO2), followed by medium removal and addition of 20 μL MTT solution (5 mg/mL) before incubating again for 4 hours at 37°C with 5% CO2. This was followed by centrifugation at 1100 rpm for 5 min and supernatant extraction. The optical density of the supernatant was recorded at 570 nm.
High-performance liquid chromatography (HPLC) fingerprint profile of VG111 (Panjab University, Chandigarh)
Fingerprinting using one of the various chromatographic techniques assures the quality and uniformity of plant-derived products, especially polyherbal products. The HPLC fingerprint of VG111 was generated using the method described in supplemental section SM3.
Clinical application: Veterinary and human application
The wound-treating efficacy of VG111 was tested by applying the formulation topically to the open wounds of the subjects. The human applications were approved under the ethics committee letters numbered IEC-1041/03.10.2020, RP-58/2020, and OP-22/04.12.2020 dated 07.12.2022 of AIIMS, Delhi. Likewise, the veterinary applications were approved under the ethics committee letter numbered V-11011(13)/16/2021-CPCSEA-DADF dated 23-09-2021 at GADVASU, Ludhiana. The use of this wound care product has been approved by the State Drug Licensing Authority (AYUSH-Ayurveda, Yoga and Naturopathy, Unani, Siddha, and Homeopathy) of India since 2020. The respective wounds were tested for microbial flora by swab sampling from deep inside the wound.
RESULTS
VG111 exhibits potent antimicrobial activity
MIC breakpoint levels of AE-VG111 against key human pathogens were observed to be between 2.5% and 5.0% (v/v) in PGIMER, Chandigarh. Excluding the S. aureus ATCC 29213 and Colistin and Polymyxin B-resistant mcr-1+ E. coli strain AR-0349, the rest of the bacterial isolates were obtained from clinical samples obtained at PGIMER, Chandigarh [Figure 1].
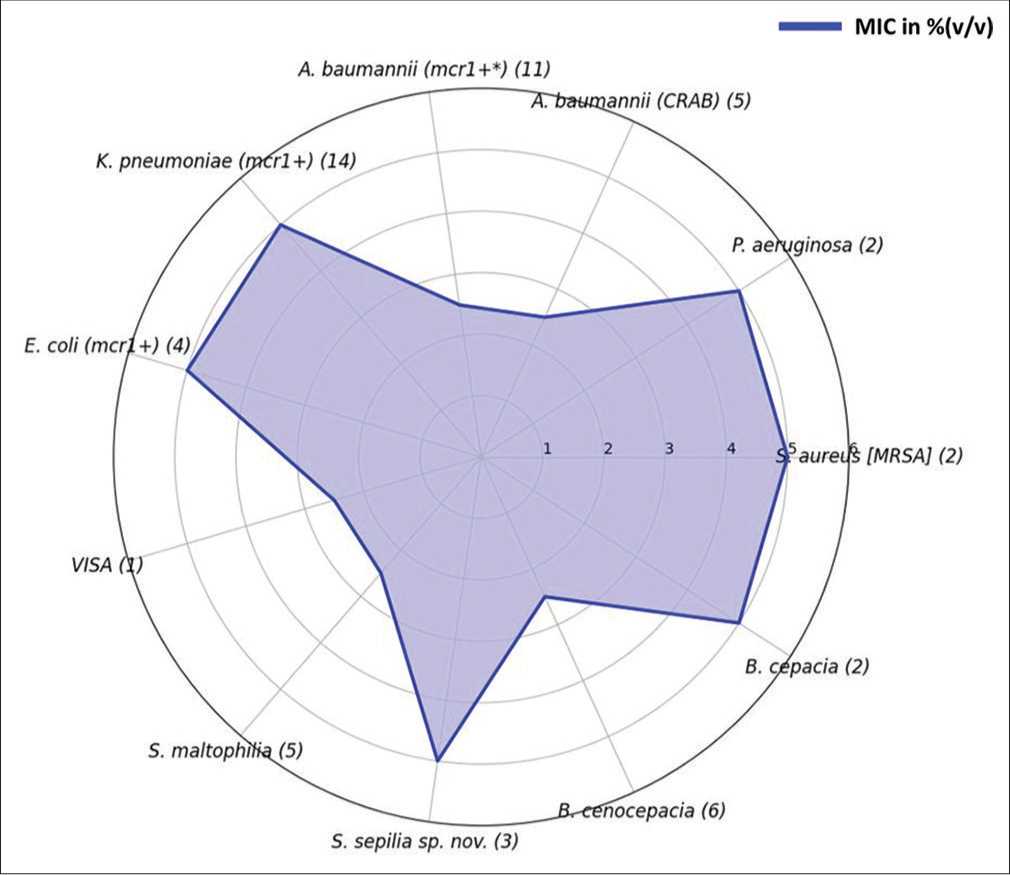
- Minimum inhibitory concentration breakpoints of AE-VG111 against medically important selected pathogens. Mcr1+ gene corresponds to colistin resistance; the number in the parentheses/brackets defines the number of isolates tested. AE-VG111: Aqueous extract of VG111. MIC: Minimum inhibitory concentration. VISA: Vancomycin intermediate S. aureus; MRSA: Methicillin-resistant S. aureus.
VG111 exhibits ROS-mediated antibacterial activity
On the evaluation of ROS activity in respective bacteria treated with AE-VG111, a dose-dependent increase in ROS was observed in the cultures of E. coli and S. sepilia. In comparison, the ROS levels in S. aureus were not as significant, although the ROS levels were similar to those observed with H2O2 treated cultures [Figure 2]. The results correlate with the viability data to the extent of cell death being directly proportionate to the ROS expression. ROS is known to increase oxidative stress and cause loss of cell membrane integrity, leading to damage to proteins and DNA in the bacteria, suggesting that the antibacterial activity of VG111 may be related to its ROS activity.
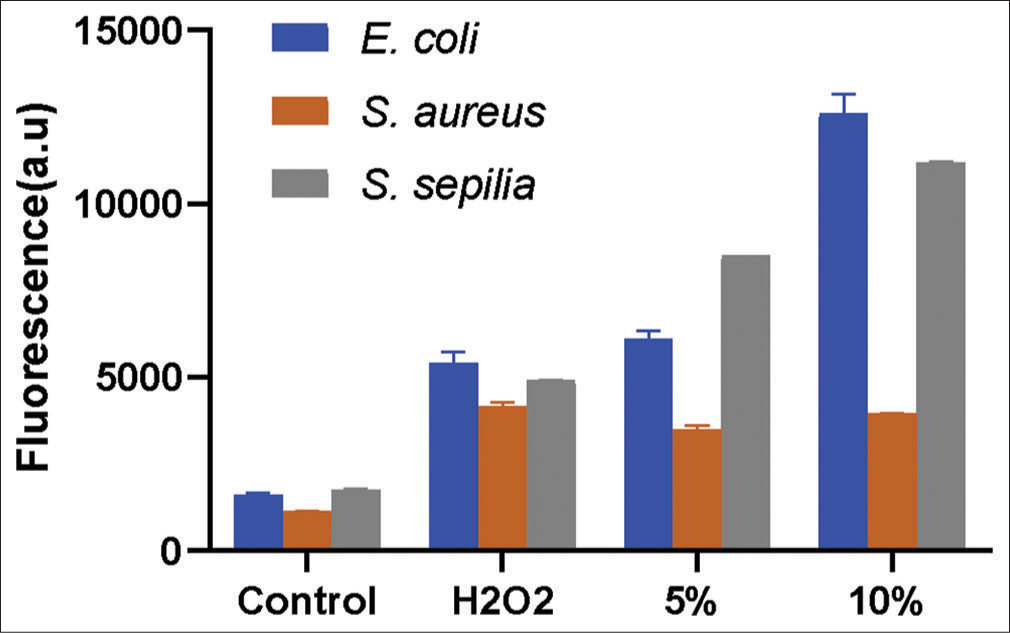
- Comparison of reactive oxygen species (ROS) generation in control and VG111 treated bacterial specimens with Hydrogen peroxide (H2O2) treatment was used as a positive control for ROS induction.
VG111 demonstrates intact biofilm disruption
AE-VG111 demonstrated remarkable anti-biofilm action on P. aeruginosa PA14 intact biofilm at a concentration of 100% (v/v). The SEM images in Figure 2 display evident biofilm clearance at varying magnifications (×5000–×20,000) for P. aeruginosa PA14 and S. sepilia SM16975 (images not shown). Note the dense slime layers covering the bacilli in the control (untreated) group and their clearance in the treated group images [Figure 3].
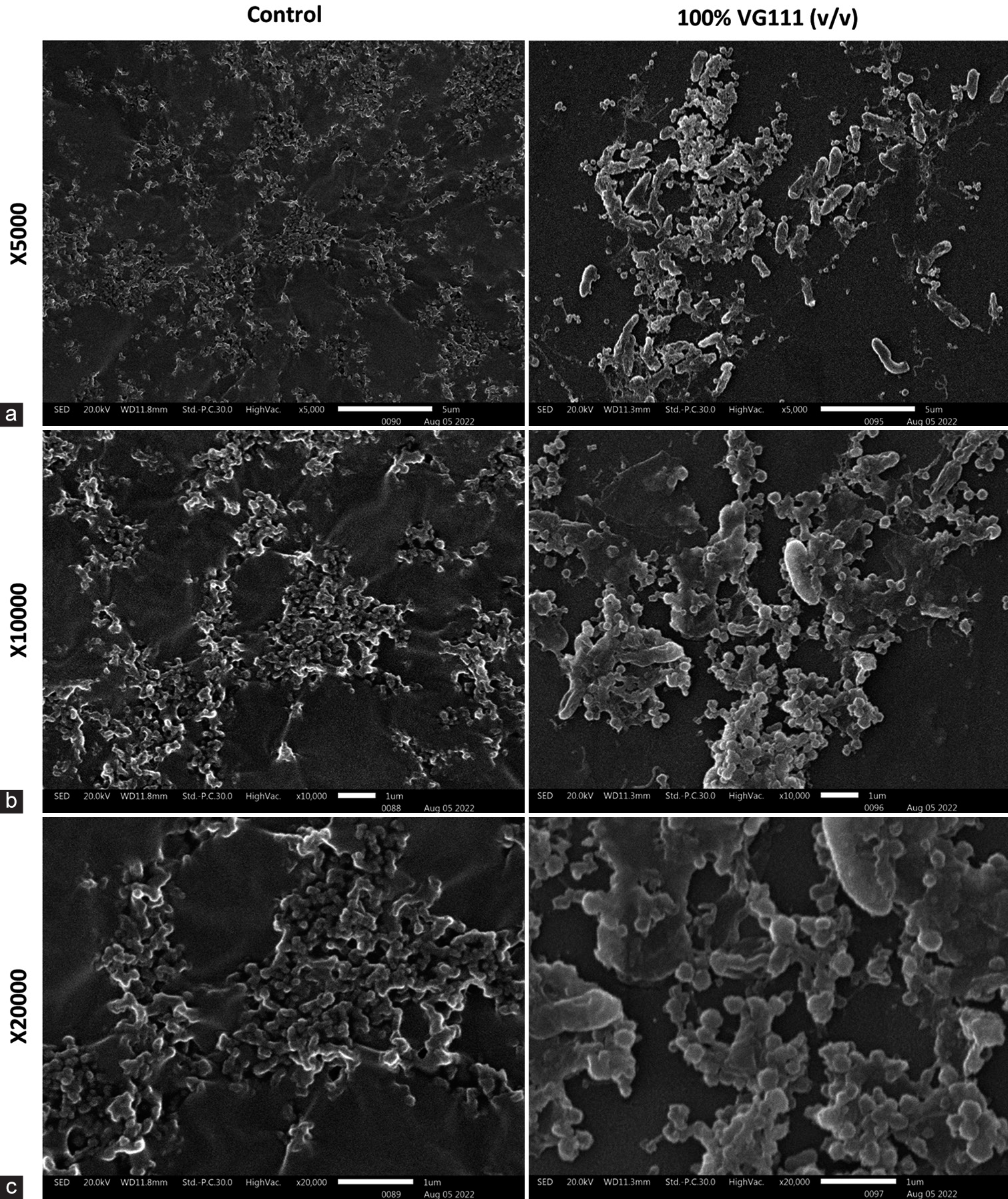
- (a-c) Scanning electron microscopy images showing comparative profile of control and 100% (v/v) AE-VG111 treated Pseudomonas aeruginosa PA14 biofilms at ×5000, ×10000 and ×20000 respective magnifications. AE-VG111: Aqueous extract of VG111.
VG111 marginally promotes fibroblast migration and wound recovery in vitro
The scratch-assay method was used to measure the in vitro wound healing properties of VG111 formulation in fibroblast cell monolayer. The quotient of fibroblast regeneration to cover the scratch wound was evaluated after 0, 6, 12, and 24 h of incubation with 2% (v/v) of aqueous VG111 formulation. Restoration of the cellular density of the fibroblasts to cover the wounded area was marginally faster in the group treated with AE-VG111 as compared with the untreated negative control group [Figure 4]. The low concentration of AE-VG111 augmented the proliferation rate of fibroblasts at different time points and stimulated the migration compared to the negative control group. Our results show the ability of VG111 to accelerate the wound healing process at low concentrations by influencing cell proliferation and maturation.
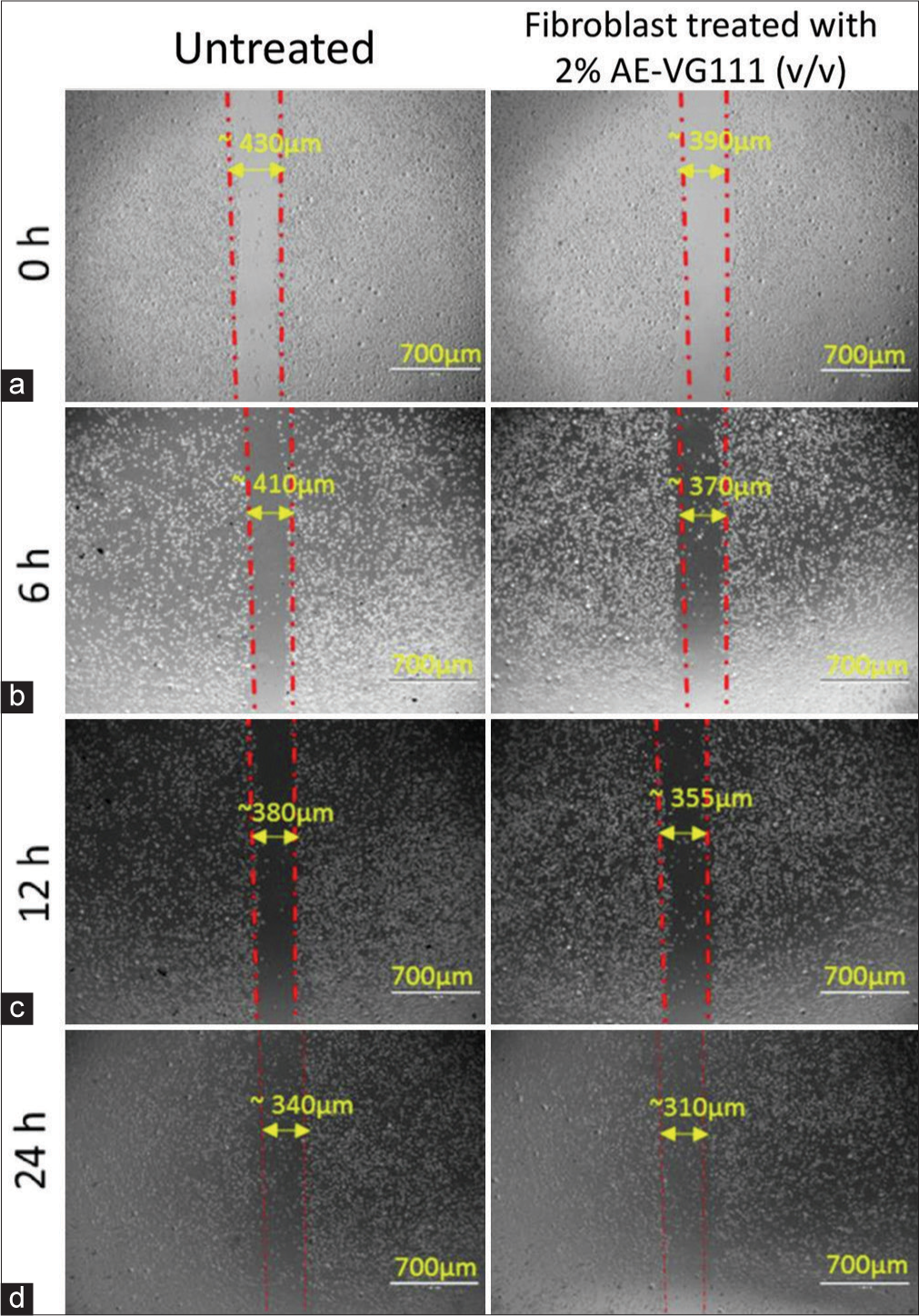
- (a-d) Scratch assay: Quantitative evaluation of in vitro cell migration by phase contrast microscopy of mouse fibroblast cell line (L929) monolayers after the creation of the scratch wound and following 0, 6, 12 and 24 h exposure to the 2% AE-VG111 (v/v) treatment. Scratch assay: Quantitative evaluation of in vitro cell migration by phase contrast microscopy of mouse fibroblast cell line (L929) monolayers after the creation of the scratch wound and following 0, 6, 12 and 24 h exposure to the 2% AE-VG111 (v/v) treatment. AE-VG111: Aqueous extract of VG111.
Marginal Suppression of pro-inflammatory cytokines level by VG111 in lipopolysaccharide (LPS)-induced mouse macrophages
Cytokines play a key role in the pathogenesis of wounds. Evaluation of cytokines in macrophages provides additional insights into the relationship between disease pathology and treatment. The level of cytokines was assessed as an indicator of inflammation and hypersensitivity in the wound. Two proinflammatory cytokines, interleukin (IL)-6, and tumor necrosis factor-alpha (TNF-a) were marginally suppressed in dose and time-dependent manner as compared to untreated control groups [Figure 5a and b]. There was no significant alteration in the levels of IL-4 and IL-10 in the treatment and control groups [Figure 5c and d].
![(a) Cytokines level in supernatant of mouse macrophages Pro-inflammatory markers interleukin-6 [IL-6] (b) & tumor necrosis factor-α [TNF-α] (c) and Anti-inflammatory markers interleukin-4 [IL-4] (d) & interleukin-10 [IL-10] after treatment with different concentrations of AE-VG111 (10, 20, and 40% [v/v]). (e) Matrix metalloproteinases levels in AE-VG111 treated mouse macrophages. Data are expressed as the mean ± standard error of mean of triplicate determinations. AE-VG111: Aqueous extract of VG111.](/content/164/2024/16/3/img/JLP-16-347-g005.png)
- (a) Cytokines level in supernatant of mouse macrophages Pro-inflammatory markers interleukin-6 [IL-6] (b) & tumor necrosis factor-α [TNF-α] (c) and Anti-inflammatory markers interleukin-4 [IL-4] (d) & interleukin-10 [IL-10] after treatment with different concentrations of AE-VG111 (10, 20, and 40% [v/v]). (e) Matrix metalloproteinases levels in AE-VG111 treated mouse macrophages. Data are expressed as the mean ± standard error of mean of triplicate determinations. AE-VG111: Aqueous extract of VG111.
Minor decrease in MMP-9 enzymes by VG111 in LPS-induced mouse macrophages
LPS markedly induced MMP-9, in supernatant of RAW464.7 mouse macrophages cell culture. In contrast, treatment of LPS-stimulated macrophages with different concentrations of VG111 prevented a slight increase in MMP-9 [Figure 5e]. Levels of MMP-9 remained near control levels despite the potent inflammatory challenge with LPS, thus demonstrating the ability of the formulation to alter macrophage function.
No cytotoxicity shown by VG111
The results from both the 24-h and 18-h assessments consistently indicate that VG111 did not induce any cytotoxic effects at the tested concentration [Figure 6]. The effect of the treatment/control group was assessed using two-way analysis of variance (Column factor, P < 0.0001). Intriguingly, the percentage viability surpassed the 100% mark with no sign of contamination, which is indicative of a proliferative perturbation on the cells.
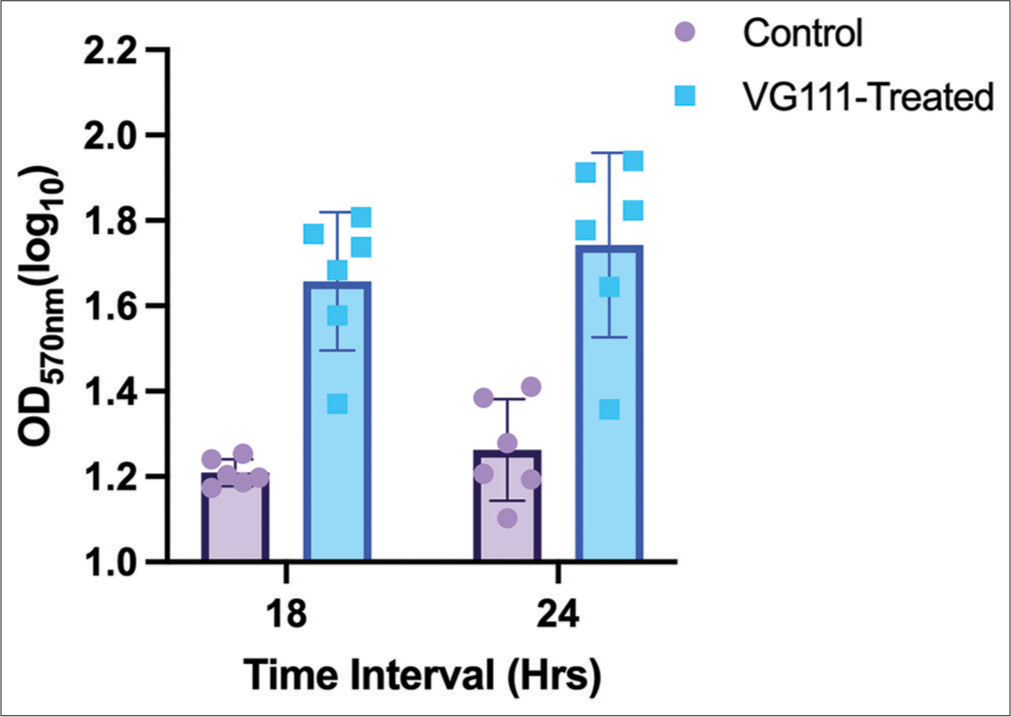
- Bar graph illustrating the impact of VG111 treatment on cell viability, as measured by optical density (OD) values, over two distinct time intervals: 18 and 24 h. Each group consists of six replicates. Error bars indicate the standard error of mean around the mean OD values.
HPLC fingerprint of VG111
The chromatogram of VG111 was obtained for aqueous extract of VG 111 [Figure 7] using C18 column and gradient mode.
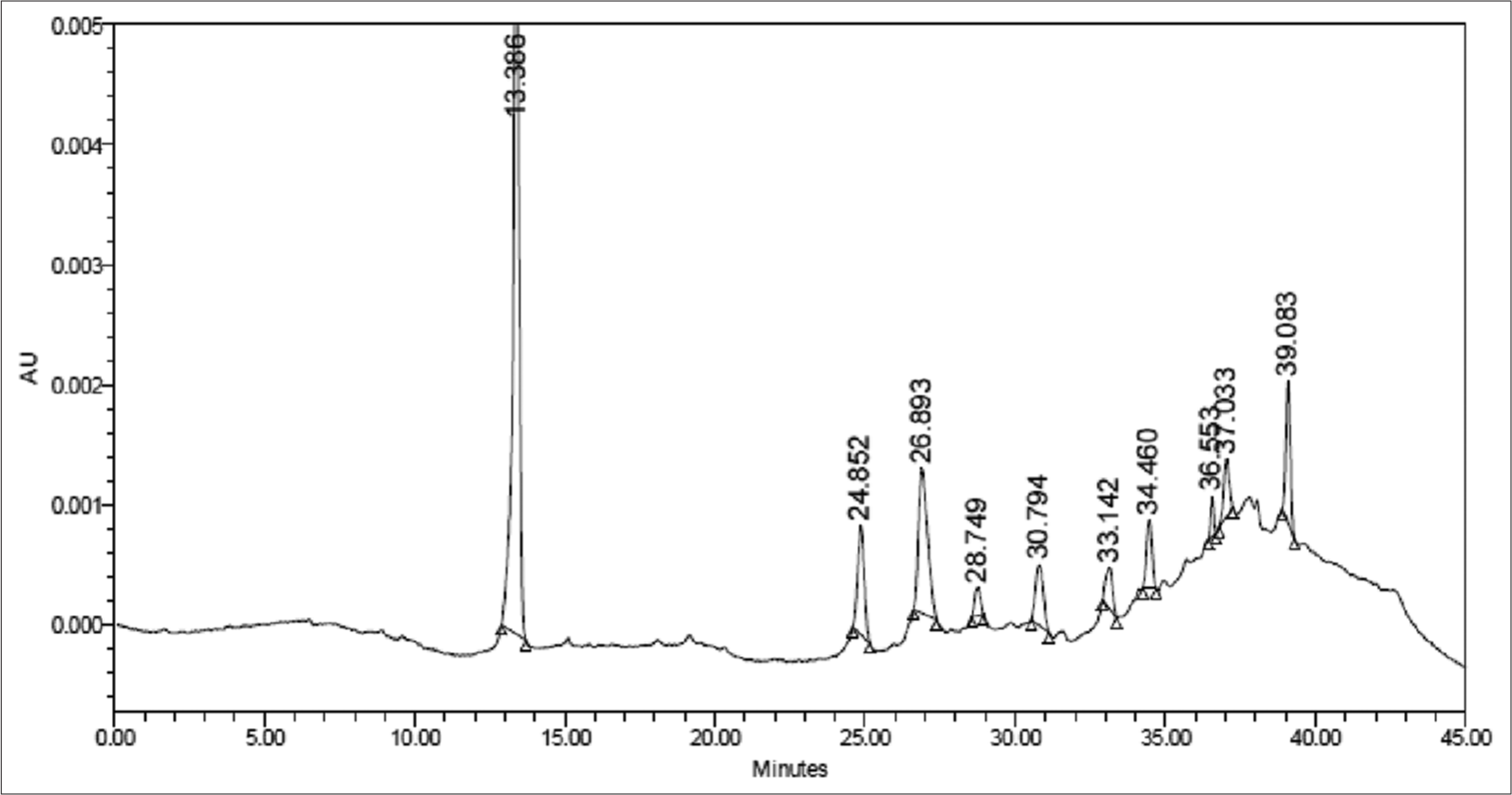
- High-performance liquid chromatography fingerprint of VG111. AU: Absorbance units.
Significant tissue repair and healing in human application
The visible wound healing effect demonstrated by VG111 oil form application in different clinical cases is shown in Figure 8.[23] The detailed case series has been discussed in the supplemental result section SR1.
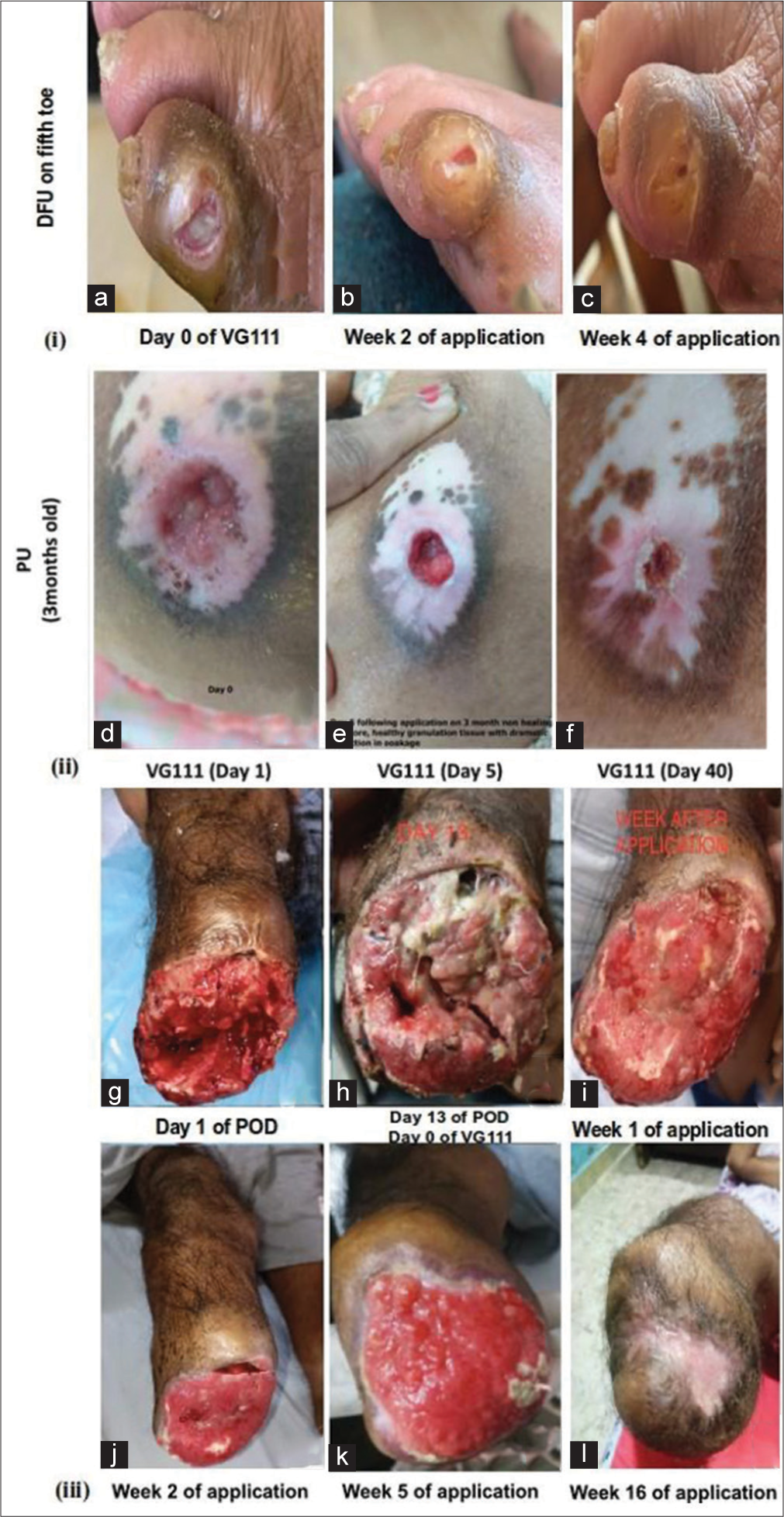
- Ulcerated wound and amputated tissue application of VG111 in humans. (i, a-c) Diabetic foot ulcer on fifth toe in 65-year-male, VG111 application was applied on 18th day following povidone-iodine based dressing; (ii, d-f) pressure ulcer on the back of 95-year female and; (iii, g-l) below-knee amputation of a 48-year diabetic male, no antibiotics or graft was required. DFU: Diabetic foot ulcers, PU: Pressure ulcers.
VG111 shows remarkable wound healing in canine applications
The efficacy of VG111 in treating wounds was tested in three dogs presented to the Teaching Veterinary Hospital out-patient department of GADVASU, Ludhiana, and showed quick healing of wounds [Figure 9]. The details of these veterinary cases have been shared in the supplemental section SR1.
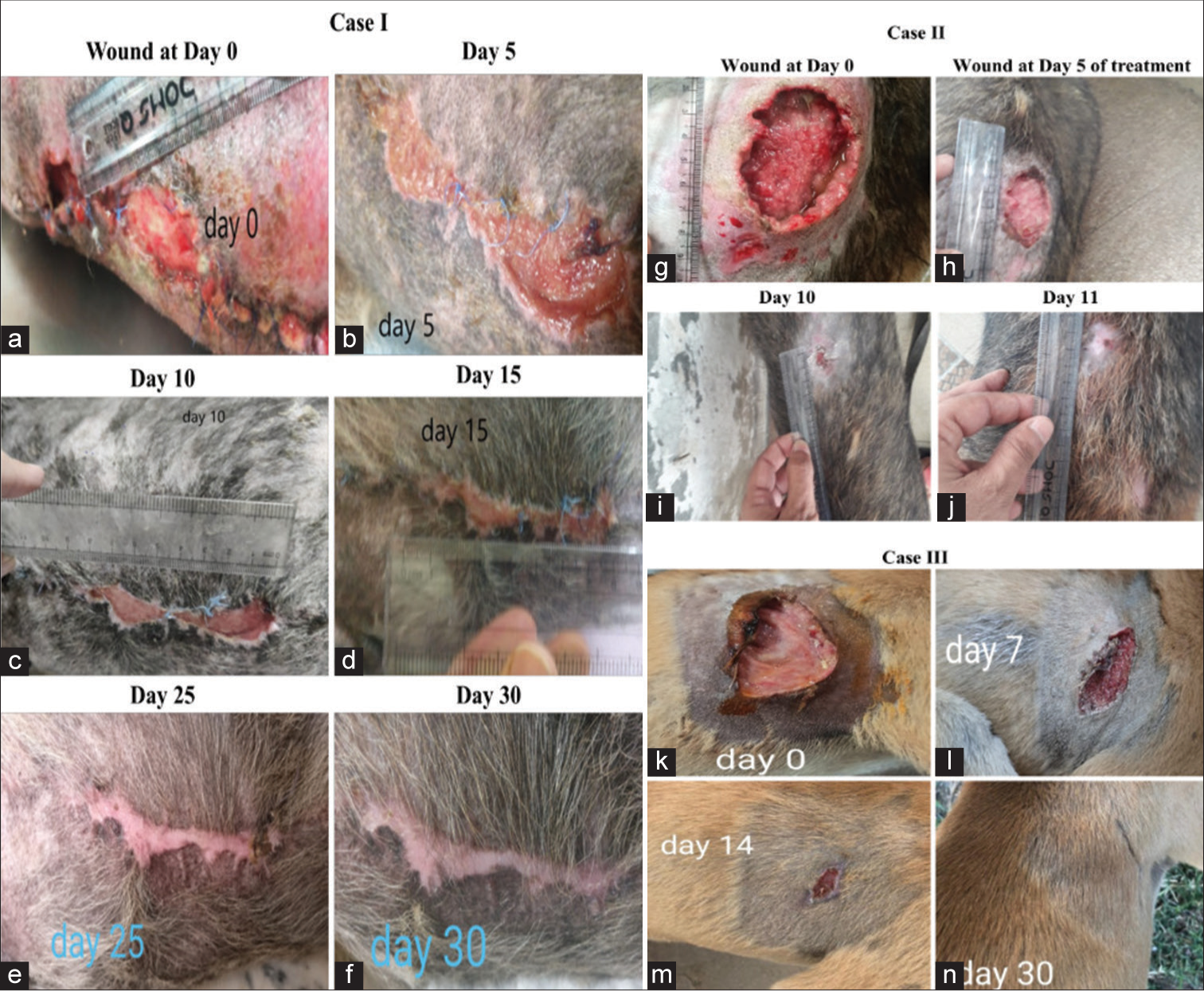
- Efficacy of topical application of VG111 on the open wounds of German shepherds (a-f) Case I, (g-j) Case II and one other indistinct dog breed (k-n) Case III. The closure of the wound orifices on 30th, 11th, and 30th day, respectively.
DISCUSSION
The present study establishes the efficacy of VG111 as a promising wound care product that is capable of addressing the present-day challenges in wound healing with a mainstay on its antimicrobial potency obviating the need of systemic antibiotics and its rapid wound healing action.
A pivotal facet of VG111’s efficacy stems from its potent antimicrobial attributes, as demonstrated across a battery of meticulously conducted experiments. The elucidation of the minimum inhibitory concentration (MIC) breakpoint through the broth microdilution assay adds precision to VG111’s antimicrobial potency. The notable range of MIC values, spanning from 2.5% (v/v) to 5.0% (v/v) across diverse pathogens, underscores VG111’s efficacy in combating microbial proliferation [Figure 1]. Particularly noteworthy is VG111’s robust action against pathogens of immense clinical relevance, such as methicillin-resistant S. aureus (MRSA), K. pneumoniae, P. aeruginosa, and Acinetobacter baumannii. The efficacy against these World Health Organization-priority list pathogens within the aforementioned concentration range accentuates VG111’s potential as a broad-spectrum antimicrobial agent. Significantly, VG111’s antimicrobial efficacy extends to pathogenic strains such as MRSA and vancomycin-intermediate S. aureus – a salient observation with direct implications for wound infection management. This becomes particularly crucial given the propensity of these pathogens to induce intractable infections in the wound microenvironment. The cumulative effect of these results underscores VG111’s potential as a formidable weapon against clinically pertinent microbial adversaries.
The observation of VG111’s capacity to disrupt P. aeruginosa PA14 biofilm represents a profound breakthrough. P. aeruginosa, a notorious biofilm-forming pathogen, contributes significantly to chronic wound infections. VG111’s influence on reducing the exopolysaccharide mass, as vividly captured in Figure 3, offers a visual representation of its efficacy in compromising biofilm integrity. The distinctive morphological alteration of bacilli following VG111 treatment, as evidenced by the images, underscores the formulation’s capacity to induce stress in biofilm-associated bacteria. This aspect acquires heightened relevance, given biofilms correlation with antibiotic resistance and the release of virulence factors in chronic wound scenarios.[25]
Transitioning toward the realm of wound healing dynamics, VG111’s impact on fibroblast behavior emerges as a significant observation. The fibroblast monolayer scratch assay provides invaluable insights into VG111-induced fibroblast dynamics. The spatiotemporal trajectory observed in Figure 4, depicting collective cell migration over time, unravels VG111’s capacity to modulate fibroblast migration – a pivotal process in wound healing. However, there does not appear to be a dramatic gap in the mensuration of the scratch area due to the selected lower concentration of 2% (v/v) of AE-VG111; a substantial variation in the rate of gap filling at regular intervals can be detected at close examination. The incremental yet discernible acceleration in gap closure over successive intervals indicates VG111’s potential to enhance fibroblast migratory responses, critically implicated in wound repair processes, especially under ulcerating conditions.
An extended exploration of VG111’s interaction with the host inflammatory response uncovers compelling trends. The inverse relationship between increasing VG111 concentration and proinflammatory cytokine levels – namely, IL-6 and TNF-a – provides insight into VG111’s potential to mitigate inflammation. Notably, this trend is not mirrored in anti-inflammatory markers IL-4 and IL-10, suggesting VG111’s role in delicately modulating the immune response [Figure 5a to d]. The nuanced interplay elucidated here forms a basis for further investigation, warranting high-resolution molecular studies utilizing precise wound fluid samples, which offer authentic prototypes of clinical wounds.
The clinical relevance of MMPs is established as a critical part of normal wound healing. In chronic wounds, levels of MMPs are elevated. The treatment of macrophages with different concentrations of VG111 in response to LPS stimulation prevented a minor rise of MMP-9, as illustrated in Figure 5e. This finding is bolstered by clinical results demonstrating VG111’s efficacy in chronic wound healing. This correlation suggests a dynamic mechanistic interplay wherein VG111’s modulation of MMPs contributes to its observed wound-healing efficacy.
Notably, VG111’s non-cytotoxic nature and its ability to stimulate cell viability, as evidenced by Figure 8, offer a profound dimension to its therapeutic profile. The intriguing observation of cell viability exceeding 100% hints at VG111’s potential proliferative effect, indicative of its influence on cellular dynamics – a pivotal insight with ramifications for wound healing cascades.
Topical formulations employed in the context of skin and wound care are typically intricate amalgamations of diverse ingredients, including the vehicle. Sesame oil served as the chosen vehicle to evaluate VG111’s effectiveness in topical application. The illustrative instances of human applications depicted in Figure 8 vividly underscore the remarkable healing prowess of VG111 across varied non-healing scenarios. In an acute DFU case [Figure 8(i)], a discernible comparison between Povidone-iodine and VG111 unveils the exacerbating impact of the former, whereas VG111 not only rectified the wound but also ameliorated the damage inflicted by Povidone-iodine. The second case, featuring a long-standing bedsore in a 95-year-old female [Figure 8(ii)], showcases notable improvement. By the 5th day of treatment, evident red granulation tissue and wound radius contraction are apparent. Subsequently, in the case of a non-healing post-below-knee amputation mass in a DFU patient [Figure 8(iii)], marked by multiple sinuses and slough production, a mere 7-day VG111 treatment triggered remarkable recovery. This was characterized by robust red granulation tissue and re-epithelialization. Microbiological analysis indicated the elimination of E. coli and the Gram-negative obligate anaerobe Bacteroides thetaiotaomicron, both originally present in intraoperative tissue specimens. Each of these human cases underscores VG111’s equivalent efficacy in fostering keratinocyte activation across distinct scenarios. Notably, canine application of VG111 demonstrates comparable wound-healing efficacy, offering a translational model for cutaneous wound studies [Figure 9]. Despite its serendipitous nature, the observed outcomes in canine cases substantiate VG111’s effectiveness, accompanied by robust hair regrowth. Notably, Case-III reveals wound healing, evidenced by a reduction in the irritated area’s perimeter and eventual wound closure within 30 days. VG111 also effectively eliminated S. aureus and P. aeruginosa burdens in these cases, reinforcing its clinical potential.
Central to VG111’s credibility is its efficacy at substantially low concentrations compared to its clinical applications. Despite the challenges posed by standardized testing methodologies for oil-based formulations, VG111’s promising outcomes underscore its potential to redefine wound care. The observed pain-relieving effects and VG111’s ability to propel wound-healing mechanisms further amplify its therapeutic significance. Notably, VG111’s multi-faceted contributions to various stages of wound healing resonate powerfully, substantiating its role as a pivotal tool in managing diabetic foot and bedsore wounds, both of which are characterized by their progressive nature.
The assessment of antimicrobial efficacy in vitro for VG111 presented challenges due to its composition as an oil-based formulation, a category for which standardized testing methodologies are notably lacking.[26] Nevertheless, a concerted effort was made in this direction, leveraging the collective expertise of a diverse team spanning multiple institutes. Remarkably, VG111 exhibited in vitro efficacy even in the absence of its vehicle, which itself holds advantageous wound-healing properties. Moreover, the experiments were conducted employing lower concentrations of VG111[26,27] adding to the robustness of the findings. Despite these constraints, the product’s promising outcomes stand out, underscoring its potential.
Noteworthy is the observation of VG111’s alleviating impact on pain in human patients within the application region, aligning with prior reports regarding specific ingredients within VG111.[28,29] The present study unequivocally demonstrates VG111’s therapeutic influence across distinct phases of wound healing. This substantiates the urgent need to advocate for VG111 as an efficacious solution in the management of diabetic foot and bedsore wounds, given their progressive nature and the pressing clinical demand for effective interventions in these contexts.
CONCLUSIONS
VG111 stands out in wound care with its strong antimicrobial action and enhanced wound recovery, effectively inhibiting key pathogens and disrupting biofilms. It supports fibroblast migration, is essential for healing, and modulates inflammation, offering promising clinical outcomes as already evident in recent case series for treating chronic wounds such as long-standing wounds and diabetic ulcers without cytotoxic concerns.
Availability of data and materials
No, all of the material is owned by the authors and/or no permissions are required.
Ethical approval
The human applications were approved under the Ethics Committee letters numbered IEC-1041/03.10.2020, RP-58/2020, OP-22/04.12.2020 dated 07-12-2020 of AIIMS, Delhi. Likewise, the veterinary applications were approved under the ethics committee letter numbered V-11011(13)/16/2021-CPCSEA-DADF dated 23-09-2021 at GADVASU, Ludhiana. The use of this wound care product has been approved by State Drug Licensing Authority (AYUSH-Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homeopathy) of India since 2020.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
Dr. Priyam Batra is on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Supplementary data available at:
Financial support and sponsorship
Nil.
References
- Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J Pharm Sci. 2020;15:661-84.
- [CrossRef] [PubMed] [Google Scholar]
- Honey: An immunomodulator in wound healing. Wound Repair Regen. 2014;22:187-92.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in surgical applications of growth factors for wound healing. Burns Trauma. 2019;7:s41038-019.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of essential oils on the process of wound healing: A review of the current evidence. J Wound Care. 2007;16:255-7.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of topical recombinant human platelet derived growth factor on wound healing in patients with chronic diabetic lower limb ulcers. Indian J Surg. 2010;72:27-31.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic wound healing: A review of current management and treatments. Adv Ther. 2017;34:599-610.
- [CrossRef] [PubMed] [Google Scholar]
- Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14:244-69.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of diabetic foot ulcer and associated factors among adult diabetic patients on follow-up clinic at JIMMA Medical Center, Southwest Ethiopia, 2019: An institutional-based cross-sectional study. J Diabetes Res. 2020;2020:4106383.
- [CrossRef] [Google Scholar]
- Prevention and treatment of pressure ulcer-a serious complication of multimorbidity and immobility. Indian J Clin Pract. 2020;31
- [Google Scholar]
- Risk factors for lower extremity amputation in patients with diabetic foot ulcers: A meta-analysis. PLOS One. 2020;15:e0239236.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of diabetes on the risk of bedsore in patients undergoing surgery: An updated quantitative analysis of cohort studies. Oncotarget. 2016;8:14516-24.
- [CrossRef] [PubMed] [Google Scholar]
- Literature review on the management of diabetic foot ulcer. World J Diabetes. 2015;6:37-53.
- [CrossRef] [PubMed] [Google Scholar]
- Perspectives in diabetes the rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51:3353-61.
- [CrossRef] [PubMed] [Google Scholar]
- The global burden of diabetic foot disease. Lancet. 2005;366:1719-24.
- [CrossRef] [PubMed] [Google Scholar]
- Health-related quality of life in diabetic patients with foot ulcers: Literature review. J Wound Ostomy Continence Nurs. 2005;32:368-77.
- [CrossRef] [PubMed] [Google Scholar]
- Management of diabetic foot problems. J Vasc Surg. 2010;51:476-86.
- [CrossRef] [PubMed] [Google Scholar]
- Wound bioburden and infection-related complications in diabetic foot ulcers. Biol Res Nurs. 2008;10:44-53.
- [CrossRef] [PubMed] [Google Scholar]
- 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-73.
- [CrossRef] [PubMed] [Google Scholar]
- A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012;20:647-57.
- [CrossRef] [PubMed] [Google Scholar]
- Opportunities and challenges of the management of chronic wounds: A multidisciplinary viewpoint. Chronic Wound Care Manag Res. 2020;7:27-36.
- [CrossRef] [Google Scholar]
- A novel potential treatment for diabetic foot ulcers and non-healing ulcers-case series. Infect Disord Drug Targets. 2024;24:29-39.
- [CrossRef] [PubMed] [Google Scholar]
- A drug-free strategy to combat bacterial infections with magnetic nanoparticles biosynthesized in bacterial pathogens. Nanoscale. 2022;14:1713-22.
- [CrossRef] [PubMed] [Google Scholar]
- Topical bacterial lipopolysaccharide application affects inflammatory response and promotes wound healing. J Interferon Cytokine Res. 2013;33:514-22.
- [CrossRef] [PubMed] [Google Scholar]
- Preparation and comparison of effects of different herbal oil ointments as wound-healing agents. Cells Tissues Organs. 2019;207:177-86.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the wound healing activity of sesame oil extract in rats. Worled J Med Sci. 2013;9:74-8.
- [Google Scholar]
- A review on natural or herbal materials and their properties used in Shalya Tantra. Himal J Health Sci. 2020;5:25-7.
- [Google Scholar]
- A review article on phytochemical and pharmacological profiles of Apamarga (Achyranthes aspera Linn) Int Ayurvedic Med J. 2015;3:2901-9.
- [Google Scholar]






