Translate this page into:
Diagnostic Performance of Commercially Available Enzyme-Linked Immunosorbent Assay Kit in the Diagnosis of Extrapulmonary Tuberculosis
Address for correspondence: Prof. Sarman Singh, E-mail: sarman_singh@yahoo.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
How to cite this article: Sankar MM, Balooni V, Singh J, Singh S. Diagnostic performance of commercially available enzyme-linked immunosorbent assay kit in the diagnosis of extrapulmonary tuberculosis. J Lab Physicians 2013;5:11-6.
Abstract
Objectives:
Antibody based serodiagnosis tests for tuberculosis (TB) was used widely in developed and developing countries. Pathozyme Myco ® immunoglobulin (Ig) M, IgA, and IgG were evaluated in pulmonary TB in many studies.
Materials and Methods:
In this study we assessed this commercially available kit in detecting extrapulmonary TB (EPTB).
Results:
A total of 354 subjects were recruited for the study, of which 217 (61.2%) were EPTB patients and 137 (38.7%) were subjects with no suggestive TB. The mean age was 29.7 ± 13.7 and 31.2 ± 15.2 years, respectively for two groups. Serum samples were tested for IgM, IgA, and IgG using Pathozyme Myco ® IgM, IgA, and IgG kit. The individual specificity rates of IgM, IgA, and IgG were 70.8% (95% confidence interval (CI): 62.7-77.7), 77.3% (95% CI: 68.6-83.5), and 68.6%. (95% CI: 60.4-75.7); while their sensitivity was 29% (95% CI: 23.4-35.4), 24.4% (95% CI: 19.1-30.5), and 34.5% (95% CI: 28.5-41.1); respectively.
Conclusion:
The serological tests either singly or in combination failed or performed poorly to diagnose EPTB.
Keywords
Diagnosis
extrapulmonary tuberculosis
serology
INTRODUCTION
The World Health Organization (WHO) declared tuberculosis (TB) a global public health emergency in 1993. TB causes ill-health among millions of people each year and ranks as the second leading infectious disease causing death worldwide. Human immunodeficiency virus (HIV) coinfection and the development of multidrug resistant (MDR) and extensively drug resistant (XDR) strains cause a major hurdle in treatment and containment of TB.[1] The latest estimates of 2011 reports that there are almost 9 million new cases and 1.4 million TB deaths of which 990,000 are among HIV negative people and 430,000 HIV-associated TB deaths.[2]
TB affects the lung which results in symptoms of respiratory system known as pulmonary TB. However, other organs, such as the pleura, lymph node, kidney, and meninges, may be involved in TB under certain circumstances and this is known as extrapulmonary TB (EPTB). Patients with EPTB present with organ-related symptoms which would develop into serious complications that threaten patients' lives and cause morbidity. Reports of recent years infer that the prevalence of EPTB is getting worse, which has drawn more public attention. In 2011, 19% of new EPTB cases were notified in India.[2] One critical and early aspect of managing the TB epidemic is early diagnosis, so that the appropriate treatment can be started at the appropriate time. Diagnosis of EPTB is challenging. Routine methods for TB diagnosis such as smear for acid-fast bacilli (AFB) and culture of Mycobacterium tuberculosis on solid media, and X-rays are poorly sensitive worldwide, nucleic acid amplification test, and liquid culture methods, such as BACTEC MGIT 960 are costly and required sophisticated infrastructure.
Due to these diagnostic limitations antibody based immunodiagnostic tests have been widely used which do not require a specimen of the affected organ for microbiological or histological examinations. There are numerous M. tuberculosis antigens, including native, semisynthetic, and recombinant ones that have been used in these diagnostic kits. Some of the antigens such as lipoarabinomannan (LAM) were commonly used and evaluated in areas with low and high burden of TB. These antibody detection methods have evolved into various formats, includes microtiter well enzyme-linked immunosorbent assay (ELISA) and immunochromatographic assays. The latter being the most widely adopted, because these do not require sophisticated instrumentation. Based on these serological methods many studies are carried out on pulmonary TB patients, while only few studies are done on EPTB patients. A meta-analysis conducted in 2007 [3] on nine published articles (25 studies) provide glimpse of variability in sensitivity (0-100%) and specificity (59-100%) of these tests. However, only one study was included from India [4] in this meta-analysis.
In 2011, World Health Organization (WHO) [5] issued strong policy recommendations against the use these antibody-based commercialized serological tests. An expert group [3,6] that reviewed the evidence on use of commercial, antibody-based serodiagnostic tests found that these tests provide inconsistent and imprecise results with highly variable values for sensitivity and specificity. No evidence was found that existing commercial serological assays improve outcomes that are important to patients.
We evaluated Pathozyme Myco ® immunoglobulin (Ig) G, IgA, and IgM (Omega Diagnostic Limited, Scotland, UK) on pulmonary TB patients from Delhi, India [7] and the findings were also highly discouraging. Simultaneously we also evaluated Pathozyme Myco ® IgG, IgA, and IgM in EPTB patients and the results are presented here.
MATERIALS AND METHODS
Setting
The study was conducted between 2007 and 2011 at the Tuberculosis Research Laboratory, Division of Clinical Microbiology and Molecular Medicine, All India Institute of Medical Sciences, New Delhi. Ethical committee of the All India Institute of Medical Sciences (AIIMS), New Delhi approved the study protocol in accordance with National Guidelines by Indian Council of Medical Research, New Delhi.
All patients with suspected EPTB were prospectively enrolled. All the demographic details and relevant clinical symptoms, signs, and duration were documented in predesigned subject information form. No subjects identified as having human immunodeficiency virus infection was included in the study.
Case definition
Enrolled patients were classified as “confirmed tuberculosis” if his sample was positive for smear/culture for M. tuberculosis; or had positive M. tuberculosis-specific DNA amplification from biological specimens; or histo-pathological findings consistent with TB or was highly suggestive radiological findings of TB (having excluded other disease) including appropriate response to antituberculosis therapy.
We defined non-TB patients as those who were bacteriologically negative for M. tuberculosis with either a resolution of clinical symptoms after an antibiotic therapy, or confirmed to have alternative diagnosis on histopathology.
Sample collection and processing
Following inclusion, a 2-4 mL venous blood sample was collected in 4 mL BD vacutainers (Backton-Dickinson, Sparks, USA) without anticoagulation and allowed it to clot at room temperature for 1 h, centrifuged at 4°C for 10 min at 3000 × g and clear serum was collected, aliquoted, and stored at −80°C till further use. No sample underwent more than one freeze-thaw cycle before analysis.
All the presumably contaminated samples were processed using modified Petroff's methods (NALC-NaOH), but samples from sterile sites such as cerebrospinal fluid (CSF), synovial fluid, bone marrow, etc., were inoculated directly in BACTEC MGIT™960 (Backton-Dickinson, Sparks, USA) and Lowenstein Jensen (LJ) medium as published elsewhere.[8] Before culture inoculation all samples were examined microscopically after Zeihl-Neelsen stain. All the isolates were confirmed as M. tuberculosis by species specific in-house multiplex polymerase chain reaction (PCR) and phenotypic methods.[9]
PATHOZYME ® MYCO IgG, IgA, and IgM test
These three assays/kits are based on two highly purified immunodominant antigens. One is cell wall lipoarabinomannan (LAM) antigen which has been proved to elicit early stage antimycobacterial immune response in some studies, and second is 38-kDa mycobacterial recombinant antigen expressed and purified from E. coli. The kits claimed to be having 91% specificity and 72% sensitivity.[10] Three immunoglobulin based ELISA kits (PATHOZYME ® MYCO IgG, IgA, and IgM) were used to check levels of antimycobacterial antibodies against two antigens in the serum of diseased and controls. The ELISA tests were performed according to the instructions in kits' manual (Omega Diagnostics Limited, Scotland, UK) and repeated three times as published earlier.[7]
Statistical analysis
For proper analysis of performance, ELISA tests were evaluated on confirmed EPTB cases and non-disease group. Sensitivity, specificity, positive predictive values (PPV), negative predictive value (NPV), and likelihood ratio for positive (LRP) test were calculated with 95% confidence intervals (CI) to determine the correct diagnosis potential of the tests. STATA SE.9 (StataCorp LP, Texas, USA) software was used for all statistical analysis.
RESULTS
Subjects and clinical characteristics
During the study period a total of 217 (61.2%) confirmed EPTB (as per the criteria mentioned in materials and methods) and 137 (38.7%) non-TB subjects were recruited. The mean age of patients with confirmed EPTB and non-TB patients was 29.7 ± 13.7 and 31.2 ± 15.2 years, respectively. Among the confirmed EPTB patients, disseminated TB was the most common (n = 75), followed by genitourinary (n = 57), lymph node (n = 37), pleural (n = 20), central nervous system (n = 13), gastrointestinal tract (n = 9), and skeletal system TB (n = 6). All subjects were examined for Bacillus Calmette-Guérin (BCG) vaccination, and 181 (83.4%) were found positive in confirmed EPTB patients and 117 (85.4%) positive in non-TB groups.
Performance of PATHOZYME ® MYCO IgG, IgA, and IgM
ELISAs were performed in all 354 subjects. The performance of all the three ELISAs were analyzed [Table 1]. The individual and overall sensitivity rates of IgM, IgA, and IgG were 29, 24.4, and 34.5%, respectively; while their specificities were 70.8, 77.3, and 68.6%, respectively. When we analyzed these in combination of two or more ELISAs, the sensitivity further decreased and ranged from 7.8 to 11.9%; but their specificities improved significantly and ranged from 83.9 to 96.3% [Table 1]. The PPV for IgM, IgA, and IgG were 61.7, 63.1, and 63.5%, respectively; whereas their NPV were 38.6, 39.2, and 39.8%, respectively. The LRP test for IgM, IgA, and IgG were 0.9, 1, and 1.1; respectively. The mean optical density (OD) values of IgM, IgA, and IgG for confirmed EPTB patients were 2.1 ± 0.7, 1.8 ± 0.8, and 1.6 ± 0.4 OD; respectively and for without TB subjects were 1.78 ± 0.6, 1.8 ± 0.9, and 1.6 ± 0.5 OD; respectively [Figure 1].
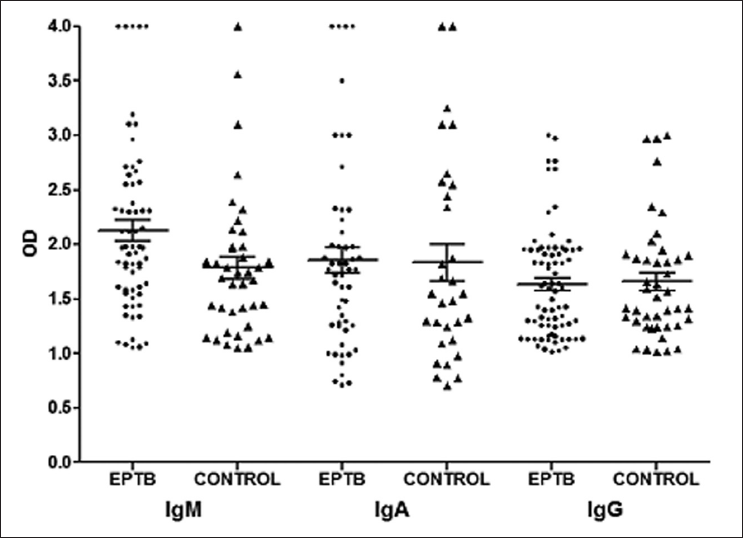
- Optical density values of IgM, IgA, and IgG positive among extrapulmonary tuberculosis (EPTB) patients without EPTB subjects
| ELISA (n=354) | Type | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | LPR (95% CI) | |
|---|---|---|---|---|---|---|---|
| EPTB (n=217) | Without EPTB (n=137) | ||||||
| IgM | |||||||
| Pos | 63 (29) | 40 (29) | 29 | 70.8 | 61.7 | 38.6 | 0.9 |
| Neg | 154 (71) | 97 (71) | (23.4-35.4) | (62.7-77.7) | (51.1-70) | (32.8-44.8) | (0.8-1.2) |
| IgA | |||||||
| Pos | 53 (24.4) | 31 (22.7) | 24.4 | 77.3 | 63.1 | 39.2 | 1.0 |
| Neg | 164 (75.6) | 106 (77.3) | (19.1-30.5) | (68.6-83.5) | (54.4-72.6) | (33.6-45.2) | (0.9-1.2) |
| IgG | |||||||
| Pos | 75 (34.6) | 43 (32) | 34.5 | 68.6 | 63.56 | 39.8 | 1.1 |
| Neg | 142 (65.4) | 94 (68.6) | (28.5-41.1) | (60.4-75.7) | (54.5-71.6) | (33.8-46.1) | (1.0-1.2) |
| IgM, IgA | |||||||
| Pos | 26 (12) | 12 (8.7) | 11.9 | 91.2 | 68.4 | 39.5 | 1.3 |
| Neg | 191 (88) | 125 (91.2) | (8.3-16.9) | (85.3-94.9) | (52.2-80.9) | (34.3-45) | (0.6-2.8) |
| IgM, IgG | |||||||
| Pos | 35 (16.1) | 22 (16) | 16.1 | 83.9 | 61.4 | 38.7 | 1 |
| Neg | 182 (83.8) | 115 (84) | (11.8-21.6) | (76.8-89.1) | (48.4-72.9) | (33.3-44.3) | (0.6-1.4) |
| IgA, IgG | |||||||
| Pos | 34 (15.7) | 15 (11) | 15.6 | 89 | 69.3 | 40 | 1.4 |
| Neg | 183 (84.3) | 122 (89) | (11.4-21.1) | (82.7-93.2) | (55.4-80.4) | (34.6-45.5) | (0.9-2.2) |
| IgM, IgA and IgG | |||||||
| Pos | 17 (7.8) | 5 (3.6) | 7.8 | 96.3 | 77.2 | 39.7 | 2.1 |
| Neg | 200 (92.2) | 132 (96.3) | (4.9-12.1) | (91.7-98.4) | (56.5-89.8) | (34.6-45.1) | (0.3-12.3) |
| Any ELISA* | |||||||
| Pos | 113 (52) | 70 (51.1) | 52 | 48.9 | 61.7 | 39.1 | 1 |
| Neg | 104 (48) | 67 (48.9) | (45.4-58.6) | (40.6-57.1) | (54.5-68.4) | (32.1-46.6) | (0.9-1) |
ELISA: Enzyme-linked immunosorbent assay, Pos: Positive, Neg: negative, CI: Confidence interval, PPV: Positive predictive value, NPV: Negative predictive value, LRP: Likelihood ratio for positive test, Ig: Immunoglobulin, EPTB: Extrapulmonary tuberculosis; *Any ELISA represents subjects detected positive by at least one of the three (IgM/IgA/IgG) ELISA tests
Performance of serology in various organ-specific disease forms
The performance of ELISA among different disease categories is shown in Table 2. The highest degree of detection for IgM was found best in central nervous system (CNS) TB (odds ratio of 2.67, 95% CI: 0.69-11.06), for IgA performed best in pleural TB (odds ratio of 2.55, 95% CI: 0.71-9.08), and IgG positive lymph node TB (odds ratio of 3.69, 95% CI: 1.02-13.34) as shown in Table 2. While comparing the detection rate among the three kits, IgG kit detected the most number of cases among lymph node TB (51.3%), gastrointestinal TB (44.4%), disseminated TB (37.3%), and bone/joint TB (33%) cases.
| Site of infection | No. of EPTB cases | IgM+ (%) | IgA+ (%) | IgG+ (%) | Any ELISA+* (%) |
|---|---|---|---|---|---|
| Bone/joint | 6 | 1 (16.6) | 1 (16.6) | 2(33.3) | 2(33.3) |
| Disseminated | 75 | 15 (20) | 14 (18.6) | 28 (37.3) | 36 (48) |
| Gastrointestinal | 9 | 3 (33.3) | 3 (33.3) | 4 (44.4) | 6 (66.6) |
| Lymph node | 37 | 15 (40.5) | 12 (32.4) | 19 (51.3) | 25 (67.5) |
| Pleura | 20 | 6 (30) | 8 (40) | 6 (30) | 12 (60) |
| CNS | 13 | 5 (38.4) | 4 (30.7) | 4 (30.7) | 6 (46.1) |
| Genitourinary | 57 | 18 (31.5) | 11 (19.2) | 12 (21) | 26 (45.6) |
+: Positive, ELISA: Enzyme-linked immunosorbent assay, OR: Odds ratio, CI: Confidence interval, CNS: Central nervous system, Ig: Immunoglobuin, TB: Tuberculosis; *Any ELISA represents subjects detected positive by at least one of the three (IgM/IgA/IgG) ELISA tests
Effect of BCG vaccination on detection rate of ELISA kit
When compared the performance of ELISA on BCG vaccination status, no statistical significance was observed neither in confirmed EPTB patients nor in non-TB groups [Table 3].
| ELISA | EPTB patients (n=217) BCG scar | Non-disease group (n=137) BCG scar | ||||
|---|---|---|---|---|---|---|
| Pos(n=181) | Neg (n=36) | P* value | Pos (n=117) | Neg (n=20) | P value* | |
| IgM | ||||||
| Pos | 51 (28.1) | 12 (33.3) | 0.5336 | 34 (29) | 6 (30) | 0.9319 |
| Neg | 130 (71.8) | 24 (66.6) | 83 (70.9) | 14 (70) | ||
| IgA | ||||||
| Pos | 43 (23.7) | 10 (27.7) | 0.6081 | 26 (22.2) | 5 (25) | 0.7838 |
| Neg | 138 (76.2) | 26 (72.2) | 91 (77.7) | 15 (75) | ||
| IgG | ||||||
| Pos | 63 (34.8) | 12 (33.3) | 0.8652 | 36 (30.7) | 7 (35) | 0.7063 |
| Neg | 118 (65.1) | 24 (66.6) | 81 (69.2) | 13 (65) | ||
| IgM, IgA | ||||||
| Pos | 22 (12.1) | 4 (11.1) | 0.8602 | 9 (7.6) | 3 (15) | 0.2868 |
| Neg | 159 (87.8) | 32 (88.8) | 108 (92.3) | 17 (85) | ||
| IgM, IgG | ||||||
| Pos | 28 (15.4) | 7 (17.2) | 0.5537 | 18 (15.3) | 4 (17) | 0.6034 |
| Neg | 153 (84.5) | 29 (80.5) | 99 (84.6) | 16 (80) | ||
| IgA, IgG | ||||||
| Pos | 29 (16) | 5(13.8) | 0.7478 | 13 (11.1) | 2 (10) | 0.8831 |
| Neg | 152 (83.9) | 31 (86.1) | 104 (88.8) | 18 (90) | ||
| IgM, IgA, and IgG | ||||||
| Pos | 14 (7.7) | 3 (8.3) | 0.9029 | 4 (3.4) | 1 (5) | 0.7275 |
| Neg | 167 (92.2) | 33 (91.6) | 113 (96.5) | 19 (95) | ||
| Any ELISA# | ||||||
| Pos | 92 (50.8) | 21 (58.3) | 0.4104 | 60 (51.2) | 10 (50) | 0.9156 |
| Neg | 89 (49.1) | 15 (41.6) | 57 (48.7) | 10 (50) | ||
*Two-tailed P value (Pearson Chi-square test); #any ELISA represents subjects detected positive by at least one of the three (IgM/IgA/IgG) ELISA tests; Pos: Positive, Neg: Negative, ELISA: Enzyme-linked immunosorbent assay, BCG: Bacillus Calmette-Guerin, EPTB: Extrapulmonary tuberculosis, Ig: Immunoglobulin
DISCUSSION
The diagnosis of active TB largely depends upon initial clinical suspicion and radiographic findings, with subsequent laboratory confirmation by bacteriologic studies. Because appropriate specimen might be difficult to obtain from extrapulmonary sites, and the number of bacilli is generally low, bacteriological confirmation of EPTB is often more difficult than pulmonary TB.[11] Even if the appropriate sample (e.g., CSF in suspected meningitis) is obtained, the sensitivity of conventional bacteriological methods are dismally poor. Because of these limitations, number of investigators relied on serology as a diagnostic tool in all settings.
With the technical advances, sets of M. tuberculosis specific antigens were first identified using either hybridoma or recombinant DNA technologies.[12] However, the findings that antibody levels are considerably higher and more frequent in the multibacillary than in paucibacillary forms of the disease [13] was noted as an obstacle to clinical application of these antigens in many studies, and thus combinations of multiple antigens were used.[14]
Here, we report poor utility of ELISA test in which the sensitivity of Pathozyme ® Myco IgM was 29%, IgA was 24.4%, and IgG was 34.5% in our patients of EPTB. These findings were by-and-large similar to our findings in pulmonary TB patients, where the sensitivity rates of the same kit were 48.7, 25.7, and 24.4%; for IgM, IgA, and IgG, respectively.[7] However, the specificity of serology was better in EPTB than pulmonary TB [Table 1]. But, the sensitivity and specificity of these kits varied among various disease forms, for example, among bone/joint TB and lymph node TB cases IgG had the highest OR of 0.83 (95% CI: 0.11-6.01), and 3.69 (95% CI: 1.02-13.34); IgA had OR of 1.6 (95% CI: 0.18-14.13), 1.25 (95% CI: 0.24-6.25), and 2.55 (95% CI: 0.71-9.08) for disseminated, gastrointestinal and pleural TB respectively, while CNS TB had the maximum OR of 2.67 (95% CI: 0.69-11.06) for IgM.
Not many authentic studies are published, but reported sensitivity of ANDA IgG detection kit used for the diagnosis of EPTB was 64% (95% CI: 28-92) for lymph node TB and 46% (95% CI: 29-63) for pleural TB, and its specificity was reported to be 90% and 87%, respectively for these patients.[3] The alternative serology method MycoDot™ is reported to have higher sensitivity of 80% and 66.7% in TB lymphadenitis and in pleural TB patients, respectively.[15] However, all studies have reported highly variable results, mainly because of difference in patient selection, criteria of diagnosis and cut-offs used. In one study, sensitivity of microwell ELISA based Pathozyme ® Myco IgM in EPTB has been reported as low as 9% [16] but in another study based on 38 kDa antigen immunochromatographic-TB test the sensitivity of 46% and specificity of 59.6% were reported in pleural TB patients.[17] Senol et al., [18] also reported sensitivity of 22.2% and 25% in pleural TB and lymph node TB, respectively; and had similar specificity of 93.3% using Pathozyme-TB Complex Plus test.
This explains the fact that not all TB patients produce antibodies against all antigenic epitopes in the cell walls of the tubercle bacilli which infers on the inconsistency in specificity of antibody based assays among different patient groups like gender, age, ethnicity, and geographical distribution. It is also well known [19] that person-to-person variation in antigen recognition is the key feature of human humoral immunity against TB.[20]
Despite the inconsistency and imprecise sensitivity and specificity of the commercial serological tests, these were being marketed liberally and used widely in major endemic regions including India until recently when WHO banned commercialization of these kits.[5] As a result of this WHO guideline, in June 2012 the Government of India stopped the import, manufacture, distribution, and sale of commercial serodiagnostic tests for TB. This bold action is expected to greatly reduce the frequency of false diagnoses of TB and facilitate the introduction of WHO approved diagnostics into the market. Therefore, in conclusion even though the specificity of the Pathozyme Myco IgA, IgM, and IgG was significantly better and acceptable as compared to pulmonary TB, the sensitivity of these kits was dismally poor and their use cannot be recommended for diagnosis of EPTB.
Source of Support:
Indian Council of Medical Research, New Delhi (ICMR Sanction Order Number: 5/8/5/4/2005.ECD.I). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest:
None declared.
ACKNOWLEDGEMENTS
Authors wish to acknowledge the technical help of Mr. Virendra Kapil (AIIMS) and assistance of Mr. Balbir Singh (AIIMS) in sample collection
REFERENCES
- High rate of extensively drug-resistant tuberculosis in Indian AIDS patients. AIDS. 2007;21:2345-7.
- [CrossRef] [PubMed] [Google Scholar]
- Global tuberculosis control. Report WHO, Geneva. Available from: http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf (accessed )
- [Google Scholar]
- Commercial serological tests for diagnosis of active pulmonary and extrapulmonary tuberculosis: An updated systematic review and meta-analysis. PLoS Med. 2011;8:e1001062.
- [CrossRef] [PubMed] [Google Scholar]
- Serodiagnosis of tuberculosis using two ELISA systems. Indian J Clin Biochem. 2003;18:48-53.
- [CrossRef] [PubMed] [Google Scholar]
- Commercial serodiagnostic tests for diagnosis of tuberculosis: Policy statement. WHO: Geneva; Available from: http://whqlibdoc.who.int/publications/2011/9792415052054_eng.pdf 2011 (accessed )
- [Google Scholar]
- Commercial serological tests for the diagnosis of active tuberculosis in India: Time for introspection. Indian J Med Res. 2011;134:583-7.
- [CrossRef] [PubMed] [Google Scholar]
- Poor performance of serological tests in the diagnosis of pulmonary tuberculosis: Evidence from a contact tracing field study. PLoS ONE. 2012;7:e40213.
- [CrossRef] [PubMed] [Google Scholar]
- 2012. Molecular characterization of Mycobacterium tuberculosis isolates from North Indian patients with extrapulmonary tuberculosis. Tuberculosis (Edinb). 2013;93:75-83.
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness of multiplex PCR in the diagnosis of genital tuberculosis in females with infertility. Eur J Clin Microbiol Infect Dis. 2012;32:399-405.
- [CrossRef] [PubMed] [Google Scholar]
- The mycobacterium tuberculosis 38-kDa antigen: Overproduction in Escherichia coli, purification and characterization. Gene. 1992;117:53-60.
- [CrossRef] [PubMed] [Google Scholar]
- Extrapulmonary tuberculosis: Management and control. In: Agarwal SP, Chauhan LS, eds. Tuberculosis control in India. New Delhi: Ministry of Health and Family Welfare; 2005. p. :95-114.
- [Google Scholar]
- Immunological study of the defined constituents of mycobacteria. Springer Semin Immunopathol. 1988;10:279-300.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of a monoclonal antibody (TB72) based serological test for tuberculosis. Clin Exp Immunol. 1983;54:337-45.
- [Google Scholar]
- Progress in serodiagnosis of Mycobacterium tuberculosis infection. Scand J Immunol. 2007;66:176-91.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the Myco Dot test for the diagnosis of tuberculosis in HIV seropositive and seronegative patients. Int J Tuberc Lung Dis. 1997;1:259-64.
- [Google Scholar]
- A comparison of seven tests for serological diagnosis of tuberculosis. J Clin Microbiol. 2000;38:2227-31.
- [CrossRef] [PubMed] [Google Scholar]
- Antibody detection for the diagnosis of tuberculous pleuritis. Int J Tuberc Lung Dis. 2001;5:968-72.
- [Google Scholar]
- Humoral immune response against 38-kDa and 16-kDa mycobacterial antigens in tuberculosis. Eur Respir J. 2007;29:143-8.
- [CrossRef] [PubMed] [Google Scholar]
- Heterogenous antibody responses in tuberculosis. Infect Immun. 1998;66:3936-40.
- [CrossRef] [PubMed] [Google Scholar]
- Assessing the serodiagnostic potential of 35 Mycobacterium tuberculosis proteins and identification of four novel serological antigens. J Clini Microbiol. 2005;43:57-65.
- [CrossRef] [PubMed] [Google Scholar]





