Translate this page into:
Temporal analysis of cryopreservation effects on human sperm phospholipase C zeta expression profile: Laboratory preliminary findings
*Corresponding author: Mona Sharma, Department of Reproductive Biology, All India Institute of Medical Sciences, New Delhi, India. dr.mona18sharma@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sharma Y, Sharma M, Halder A, Mahey R, Kumar N, Dipankar S. Temporal analysis of cryopreservation effects on human sperm phospholipase C zeta expression profile: Laboratory preliminary findings. J Lab Physicians. 2024;16:328-37. doi: 10.25259/JLP_28_2024
Abstract
Objectives:
The objectives of this study were to compare the sperm parameters and sperm phospholipase C zeta (PLC ζ) expression profile before and after vitrification.
Materials and Methods:
Pre-vitrification and post-vitrification analysis of semen samples of 14 infertile men was carried out for sperm parameters such as motility, vitality, and morphology by standard semen analysis procedures, whereas the proportion of sperms exhibiting PLC ζ and its localization pattern was assessed by indirect immunofluorescence. The temporal analysis was done thrice: over 1 week, 1 month, and 3 months of cryostorage.
Statistical Analysis:
Statistical analyses were performed using GraphPad Prism version 8.0.1 (San Diego, California, USA). Data were expressed as mean ± standard deviation. Parametric or non-parametric tests were employed for comparisons according to the normality of data distribution and the available data set. P value of 0.05 was considered statistically significant.
Results:
Vitrification was found to be associated with a decrease in the percentage of sperm motility (P ≤ 0.0001), vitality (P ≤ 0.0001), spermatozoa exhibiting normal morphology (P > 0.05), and PLC ζ protein (P > 0.05), however, the latter two, only, insignificantly. There was increased dominance in the post-acrosomal localization of PLC ζ after vitrification (P ≤ 0.001).
Conclusions:
The post-acrosomal localization of PLC ζ has been reported to have the highest positive correlation with oocyte fertilization and the present study showed the predominant pattern of the same. The implications for quality maintenance for long storage periods can be suggested as better sperm quality was observed at 3 months of storage during this study. This raises the hypothesis that the vitrification method of sperm cryopreservation may be the method of choice for routine clinical use in the assisted reproductive technology settings.
Keywords
Sperm cryopreservation
Vitrification
Phospholipase C zeta
Sperm oocyte activation factor
INTRODUCTION
Sperm cryopreservation is a routine technique in assisted reproductive technology (ART) programs employed to preserve an individual’s reproductive potential under conditions when their fertility is at risk or as a part of their infertility treatments.[1,2] Cryopreservation of sperms is an important cornerstone for all Assisted Reproductive Laboratory procedures but are associated with inevitable damage to the sperm including its proteins. The phospholipase C zeta (PLC ζ) protein, a putative sperm oocyte activation factor (SOAF), has well implicated roles in successful fertilization. The cryopreservation of human spermatozoa can be achieved through two principal approaches: slow-rate freezing and rapid freezing.[1] Slow freezing is the conventional method whereby sperm is frozen by progressive cooling using very slow cooling rates, either manually or automatically, involving the use of cryoprotectants.[3] On the other hand, the novel ultra-fast method called vitrification involves instant sperm cooling using rapid cooling rates during which water solidifies as an amorphous glass-like structure instead of ice. Vitrification is time and cost-efficient requiring no special equipment, plus has been suggested to be less traumatic to cells by preventing cell shrinkage and osmotic cell damage.[4,5] Vitrification, by directly plunging the sperm samples into liquid nitrogen (LN2), is thus considered as a promising standard method of cryopreservation for gametes including sperms.[6,7]
Regardless of the method chosen for cryopreservation, most cells are known to inevitably undergo some form of damage, including vitrification.[8] Damage to sperm acrosome, cell membrane, DNA integrity, mitochondria, cytoskeleton, sperm proteins, and adverse effect on sperm motility, morphology, and viability have been adequately reported post-cryopreservation.[9-15]
The inexorable cryoinjuries in cells have been linked to reduced fertilization capacity of spermatozoa in post-thaw samples. Although in-vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) are currently the most efficient variants of assisted fertilization for the treatment of mild and severe male infertility, respectively, fertilization failure and abnormal fertilization are a common scenario. Complete fertilization failure still occurs in 1–5% of ICSI cycles.[16,17]
Besides technical errors such as incorrect injection, most studies on the etiology of fertilization failure after ICSI reveal that the predominant cause is oocyte activation failure due to a deficiency of SOAF. The most studied SOAFs are PLC ζ, citrate synthase, etc.[18,19]
SOAFs are released into the ooplasm, post-fusion of the sperm and ovum. SOAFs are needed to release oocyte metaphase arrest by downstream PLC ζ-mediated signaling pathways.[20,21] Many sperm proteins have been hypothesized as oocyte activation factors due to their activity; however, the role of testis-specific 70 Kda PLC ζ has been found most promising.[22] A battery of evidence supports the role of PLC ζ in oocyte activation inducing Ca2+ oscillation in bovine and murine oocytes, followed by microinjection of PLC ζ complementary RNA (cRNA) or recombinant protein.[23] Further, it has also been found that injection of human PLC ζ cRNA and recombinant PLC ζ protein into human oocytes triggers calcium oscillations and development to the blastocyst stage.[24] Given the evidence implicating PLC ζ as the endogenous agent of oocyte activation, it seems plausible that failed ART cycles are due to a defect in PLC ζ structure or function post-cryopreservation. Understanding the temporal effect of the novel vitrification method of sperm cryopreservation on the activation factor PLC ζ is thus necessary.
MATERIALS AND METHODS
The cases selected for the study were of idiopathic male infertility and referred from the fertility clinic (IVF unit) in the Department of Obstetrics and Gynecology at All India Institute of Medical Sciences, New Delhi. The protocol of the study was approved by the Institute Ethics Committee (IECPG-28/February 27, 2020) and written informed consent was taken from all enrolled patients. Semen samples were obtained from 14 infertile men by masturbation after 2–7 days of sexual abstinence.
Semen analysis
A freshly collected semen sample was allowed to liquefy for at least 30 min at 37°C in the incubator. After the liquefaction of the sample, semen analysis was performed according to the specifications of the WHO laboratory manual for the Examination and Processing of Human Semen (2010). Semen samples were evaluated for volume, pH, concentration, motility, vitality, and morphology. Sperm count was assessed using an improved Neubauer’s chamber, whereas vitality was by Eosin-Nigrosin staining. At least 200 spermatozoa were analyzed for the evaluation of motility, concentration, and vitality. Sperm morphology was assessed in at least 100 spermatozoa by Papanicolaou’s stain.
Sperm preparation
Samples were prepared by the swim-up technique previtrification. After complete liquefaction, 1 mL of semen was placed in a conical tube and centrifuged at 1500 rpm for 5 min. Post-centrifugation, the supernatant was carefully discarded, and the pellet was layered with 1–1.5 mL fresh medium depending on the pellet size. The tube was then incubated at an angle of 45° at 37°C for 1 h time. The upper layer, rich in motile sperm was collected carefully, following which sperm motility, vitality, morphology, and PLC ζ positive sperms were assessed.
Sperm vitrification
After processing through the swim-up technique, sperm suspensions were divided into aliquots. Each aliquot was gently mixed with an equal volume of sperm-freezing solution (Vitrolife, Gothenburg, Sweden), which contained glycerol as a cryoprotectant and cholesterol for additional membrane protection. This medium was added dropwise to the suspension and then carefully tilted after each drop was added and incubated at room temperature for 10 min according to the manufacturer’s instructions. This semen mixture was then subdivided into further aliquots.
Direct cryovial plunging was used for sperm freezing. 1.5 mL cryovials for each sample were marked with the identification number, and the semen mixture, in 100 mL volume each was loaded into respective cryovials. The cryovials were then quickly plunged into the LN2 and stored at −196°C until analysis. The parameters were checked after thawing at 1-week, 1-month, and 3-month intervals.
For warming, the cryovials were removed from −196°C and placed in a water bath at 35 ± 2°C for 10 min. The semen mixture was transferred to a fresh tube and then diluted with an equal amount of equilibrated gamete handling medium (G-GAMETE™, Vitrolife, Gothenburg) in a drop-wise manner with adequate mixing. The mixtures were centrifuged at 300 g for 6 min to remove the freezing solution containing the cryoprotectant. The tubes were then incubated at 37°C in 5% CO2 for at least 10 min before analysis.
Immunofluorescence of PLC ζ
The samples were treated based on the procedure described by Yelumalai et al. and Grasa et al.[23,24] Briefly, the sample was pelleted by centrifugation at 1500 g for 5 min. After a single wash in Phosphate buffer saline (PBS), sperms were fixed with 4% paraformaldehyde for 30 min at room temperature followed by two subsequent washing. The sample was then diluted as appropriate and drawn on slides, after which it was permeabilized with 0.5% Triton-X 100 (in PBS) for 30 min. Post rinse with PBS, blocking was carried out with 3% bovine serum albumin (BSA), and primary antibody (25 mg/mL in PBS/0.05% BSA) was applied and left overnight at 4°C. It was then washed thrice with PBS and incubated with a secondary antibody (5 mg/mL) for 1 h at room temperature. Following washing with PBS, 10 mL of 4’,6-diamidino-2-phenylindole (DAPI) solution was applied in the dark for 15 min at 37°C. Three subsequent washings were done, and the slides were mounted with the antifade solution. Samples were observed under a fluorescence microscope (Eclipse E400, Nikon) at ×100 magnification using a fluorescein isothiocyanate filter (excitation filter: 450–490 nm; dichroic mirror: 505 nm; and barrier filter: 515–550 nm) specific for green fluorescence (excitation wavelength: 488 nm). At least 100 spermatozoa were evaluated per sample. The percentage of spermatozoa exhibiting PLC ζ, as well as the localization profile in each spermatozoon was evaluated. Each analyzed spermatozoon was classified into one of the following five categories according to PLC ζ localization: acrosomal (A); post-acrosomal (PA); equatorial (E); acrosomal and equatorial (A+E); post-acrosomal and equatorial (PA+E); or “none” indicating a total absence of PLC ζ.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 8.0.1 (San Diego, California, USA). Data were expressed as mean ± standard deviation. Parametric or non-parametric tests were employed for comparisons according to the normality of data distribution and the available data set. P < 0.05 was considered statistically significant.
RESULTS
A total of 14 samples from men with idiopathic infertility were collected and analyzed. Seven of the ejaculates exhibited normal semen parameters, whereas the remaining seven samples exhibited subnormal parameters having at least one sperm parameter less than the lower reference limit. The data on the initial ejaculate quality are summarized in Table 1. On preparation, all three parameters, namely, motility, vitality, and morphology showed improvement on an average of 8%, 7%, and 2%, respectively. The effect of vitrification among normal (n = 7) and subnormal (n = 7) samples was compared from neat unprepared sperm quality whereas mean sperm parameters (n = 14) were compared for vitrification effect after sperm preparation (pre-vitrification).
| Sperm parameters | Sperm concentration (106/mL) | Sperm count (106) | Sperm motility (%) | Sperm vitality (%) | Sperm morphology (%) |
|---|---|---|---|---|---|
| Normal (n=7) | 102.34×±54.76 | 258.34±148.85 | 64.86±0.09 | 69.14±0.07 | 15.57±0.09 |
| Subnormal (n=7) | 43.64±47.18 | 86.88±118.19 | 52±0.14 | 58.43±0.12 | 7.71±0.08 |
Effect of vitrification on sperm motility
The mean average motility in normal ejaculates (n = 7) was 65%, whereas the mean average motility in subnormal ejaculates (n = 7) was 52% (P ≥ 0.05). Motility showed a similar trend for both normal and subnormal samples: A rapid decrease in the 1st week (−30% for normal [P ≤ 0.001] and −36% [P ≤ 0.001] for subnormal), a further decrease in the 1st month (−8% in normal (P ≤ 0.05), and −6% [P ≤ 0.05] in subnormal), whereas an increase in the 3rd month (+6% in normal [P ≤ 0.05] and +5% [P ≤ 0.05] in subnormal).
The overall decrease in 3 months for normal samples was 32% (P ≤ 0.001), whereas 37% (P ≤ 0.0001) for subnormal samples. All changes for both types of samples were found to be statistically significant [Table 2 and Figure 1]. Interestingly, it was found that the decrease in motility of subnormal samples was significantly greater than in the normal samples at all time points.
| Sperm Parameters | Pre vitrification | Average change, Post vitrification | Net change | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Subnormal | Week 1 | Month | Month 3 | Normal | Subnormal | ||||
| N | SN | N | SN | N | SN | |||||
| Motility | 65% | 52% | −30% | −36% | −8% | −6% | +6% | +5% | −32% | −37% |
| Vitality | 69% | 58% | −50% | −47% | −4% | −3% | −1% | nc | −55% | −50% |
| Morphology | 16% | 8% | −3% | −3% | nc | nc | +3% | +3% | nc | nc |
| PLC ζ | 51% | 37% | −10% | −11% | −2% | +1% | +7% | +6% | −5% | −4% |
N: Normal samples; SN: Subnormal samples; nc: No change; PLC ζ: Phospholipase C zeta
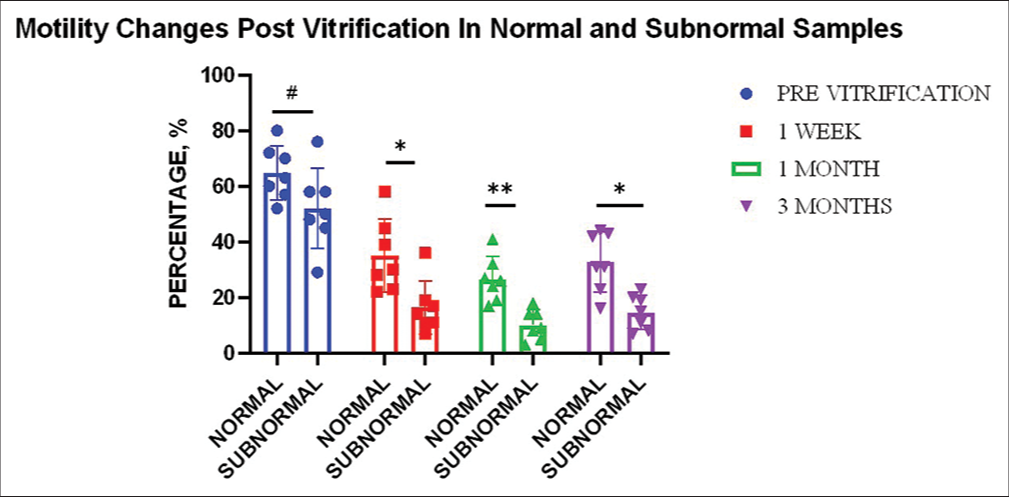
- Effect of vitrification on sperm motility for different sample types (n = 7). Graph indicates significant change with respect to normal and subnormal samples in motility post 1 week (P ≤ 0.05)*, 1 month (P ≤ 0.01)**, and 3 months (P ≤ 0.05)* of vitrification compared to the pre-vitrification status of neat samples. “#” indicates non-significant change (P ≥ 0.05).
Overall, a marked decrease was observed in the total motility (n = 14) in post-vitrified samples. The decrease was highly evident in the 1st week of preservation whereby there was a reduction in motility by an average of 41%. A further decrease of 8% was seen in the 1st month, whereas a 6% increase was noted in the 3rd month. The net decrease at the end of 3 months accounted to −43%. Reduction at all-time points as compared to the motility before vitrification was found to be statistically significant. The change in motility between time periods was, however, found to be insignificant (P ≥ 0.05) [Figure 1].
Effect of vitrification on sperm vitality
The mean average viability in normal ejaculates (n = 7) was 69%, whereas the mean average viability in subnormal ejaculates (n = 7) was 58% (P ≥ 0.05). Vitality analysis showed a similar trend for both normal and subnormal samples: A rapid decrease in the 1st week (−50% for normal (P ≤ 0.0001) and −47% (P ≤ 0.0001) for subnormal), a further decrease in the 1st month (−4% in normal (P ≥ 0.05), and −3% (P ≥ 0.05) in subnormal) while a minor decrease of 1% (P ≥ 0.05) in the 3rd month for normal samples, whereas no average decrease for subnormal samples (P ≥ 0.05). The overall decrease in 3 months for normal samples was −55% (P ≤ 0.0001) whereas −50% for subnormal samples (P ≤ 0.0001) [Table 2]. The decrease in the 1st week and the overall change for both types of samples was found to be highly significant statistically. It was found that the change in vitality between normal and subnormal samples did not significantly differ before vitrification, during the 1st week or 1st month. However, a significant difference (P ≤ 0.05) in change between the two groups was detected in the 3rd month of vitrification [Figure 2].
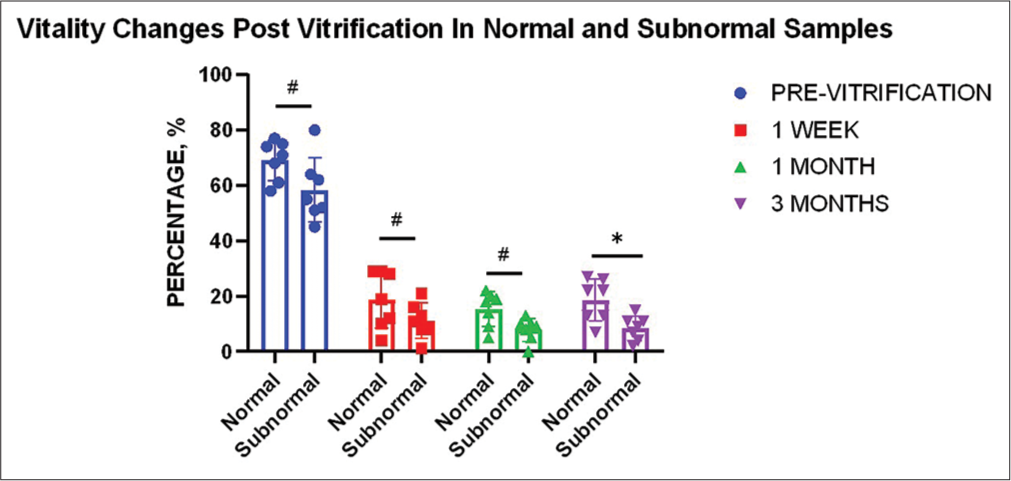
- Effect of vitrification on sperm vitality for different sample types (n = 7). Graph indicates a significant change between normal and subnormal samples only in the 3rd month (P ≤ 0.05)* of vitrification compared to the pre-vitrification status of neat samples. “#” indicates non-significant change.
Overall, a marked decrease was observed in sperm viability (n = 14) with an average decrease of 56% in 1 week, with a further 3% decrease in 1 month and no further change in the 3rd month. The net decrease at the end of 3 months accounted to −59%. Reduction at all time points as compared to the vitality before vitrification was found to be statistically significant (P ≤ 0.0001). The change in vitality between time periods was however found to be insignificant (P ≥ 0.05).
Effect of vitrification on sperm morphology
The mean average morphology in normal ejaculates (n = 7) was 16%, whereas the mean average morphology in subnormal ejaculates (n = 7) was 8% (P ≥ 0.05). Morphology analysis showed a very similar trend for both normal and subnormal samples: A decrease of 3% in the 1st week, no further decrease in the 1st month, whereas an increase of 3% in the 3rd month [Table 2]. No change at any time points for both types of samples was found to be statistically significant (P ≥ 0.05). On statistical testing, it was found that the change in morphology regarding normal and subnormal samples did not significantly differ at any time points (before vitrification, during 1st week, 1st month, or 3rd month) [Figure 3].
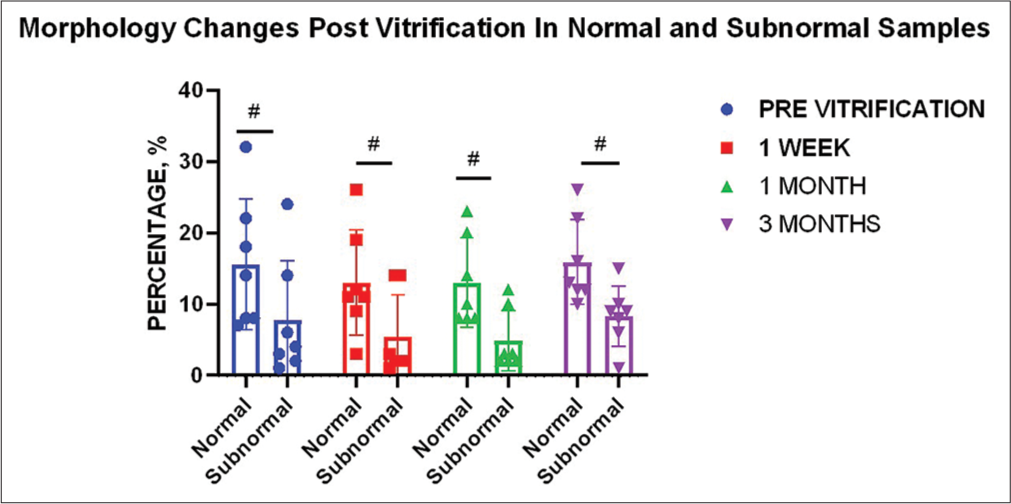
- Effect of vitrification on sperm morphology for different sample types (n = 7). Graph indicates no significant change between normal and subnormal ejaculates at all time points compared to the pre-vitrification status of neat samples.“#” indicates non-significant changes.
Overall, an average decrease of 5% in morphology (n = 14) was seen in the 1st week of cryopreservation. No further average decrease was seen in the 1st month, whereas an increase of 3% was evident in the 3rd month. The net decrease over the period of 3 months was concluded to be 2%. However, changes at all time points as compared to the percent normal morphology before vitrification were found to be statistically insignificant. No significant change in morphology was found between time periods also.
Effect of vitrification on PLC ζ
The average PLC ζ positive sperms in normal ejaculates (n = 7) was 51%, whereas the average PLC ζ positive sperms in subnormal ejaculates (n = 7) was 37% (P ≥ 0.05).
Immunofluorescence analysis showed a similar trend for both normal and subnormal samples: A decrease in the 1st week (−10% for normal ejaculates [P < 0.01]; −11% [P < 0.01] for subnormal ejaculates), a little change in the 1st month (−2% for normal [P ≥ 0.05]; +1% [P ≥ 0.05] for subnormal), whereas an increase in the 3rd month for both (+7% for normal [P ≥ 0.05]; +6% for subnormal [P ≥ 0.05]) [Table 2]. There was no significant change seen regarding normal and subnormal samples at all time points compared to the pre-vitrification status (P ≥ 0.05).
Overall, the percentage of spermatozoa exhibiting PLC ζ (n = 14) was also seen to decrease after thawing, although not significantly. In the 1st week, the percentage of PLC ζ-positive sperm decreased from an average of 44% to 34% and decreased by only a further percent in the 1st month. However, there was an increase in the finding of PLC ζ-positive sperms by 6% by 3rd month. The net decrease at the end of 3 months accounted to −6% [Figure 4]. However, changes at all time points, compared to the percent PLC ζ-positive sperms before vitrification, were found to be statistically insignificant. Furthermore, no significant change was evident in the percentage of spermatozoa exhibiting PLC ζ between time periods. On checking the correlation between PLC ζ and other sperm parameters under study, it was seen that changes in PLC ζ on vitrification were significantly correlated with the changes in morphology at 1 week (P ≤ 0.01), 1 month (P ≤ 0.05), and 3 months (P ≤ 0.01) [Table 3].
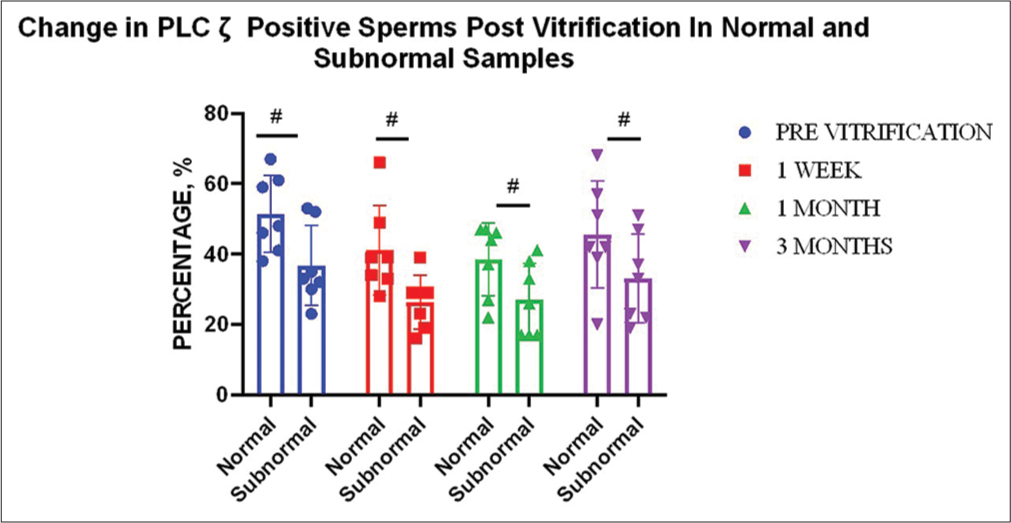
- Effect of vitrification on percent: phospholipase C zeta (PLCζ) positive sperm for different sample types (n = 7). Graph indicates no significant change with regard to normal and subnormal samples at all time points compared to the previtrification status.“#” indicates non-significant changes.
| Motility and PLC ζ | Vitality and PLC ζ | Morphology and PLC ζ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coeff. | P-value | Significance | Coeff. | P-value | Significance | Coeff. | P-value | Significance | |
| 1 week | 0.462 | 0.096 | ns | 0.548 | 0.087 | ns | 0.765 | 0.001 | ** |
| 1 month | 0.384 | 0.989 | ns | 0.384 | 0.174 | ns | 0.584 | 0.028 | * |
| 3 months | 0.083 | 0.777 | ns | -0.042 | 0.886 | ns | 0.715 | 0.004 | ** |
Coeff.: Correlation coefficient; ns: Non-significant; PLC ζ: Phospholipase C zeta; *indicate p<0.05 and **indicate p<0.01
Localization of PLC ζ
Although the localization of PLC ζ was found to vary from sperm to sperm within and across samples, the dominant localization pattern overall was found to be equatorial position (54.69% in the fresh sample, 36.65% in 1st week, 30.50% in 1st month, and 30.98% in 3 months). In the fresh sample, the dominant pattern was equatorial followed by acrosomal and equatorial and equatorial and post-acrosomal localization, respectively (17.31% and 17.15%) [Photomicrograph 1].
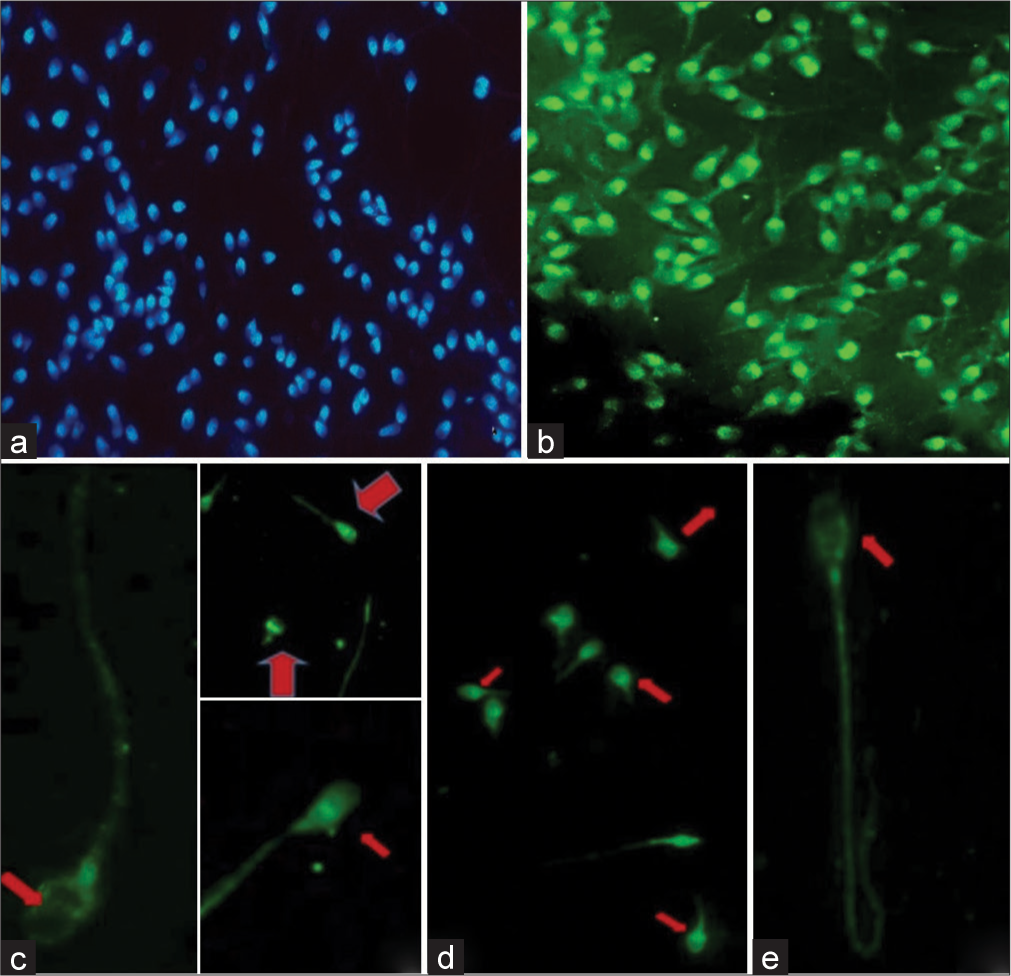
- Immunofluorescence staining of sperms for phospholipase C zeta (PLC ζ). (a) 4’,6-diamidino-2-phenylindole (DAPI)-stained nuclei of sperm, (b) Sperm cells under FITC filter showing green fluorescence specific for PLC ζ, (c) Localization of PLC ζ in the equatorial region (red arrow) of sperm head, (d) Localization of PLC ζ in the post-acrosomal region of sperm head (red arrows), (e) Localization of PLC ζ in the equatorial + post-acrosomal region (red arrow) of sperm head.
Post-warming, however, the localization pattern of the PLC ζ protein was found to be altered. With time, the equatorial dominance decreased (proven significant), whereas the post-acrosomal localization showed an increasing trend [Figure 5]. A significant decrease in PLC ζ equatorial localization post 1 week, 1 month, and 3 months of vitrification compared to the pre-vitrification status was evident. On the other hand, a significant increase (P ≤ 0.01 and P ≤ 0.05) in PLC ζ post-acrosomal localization post 3 months of vitrification compared to the pre-vitrification and post-1-week status was seen, respectively [Figure 5].
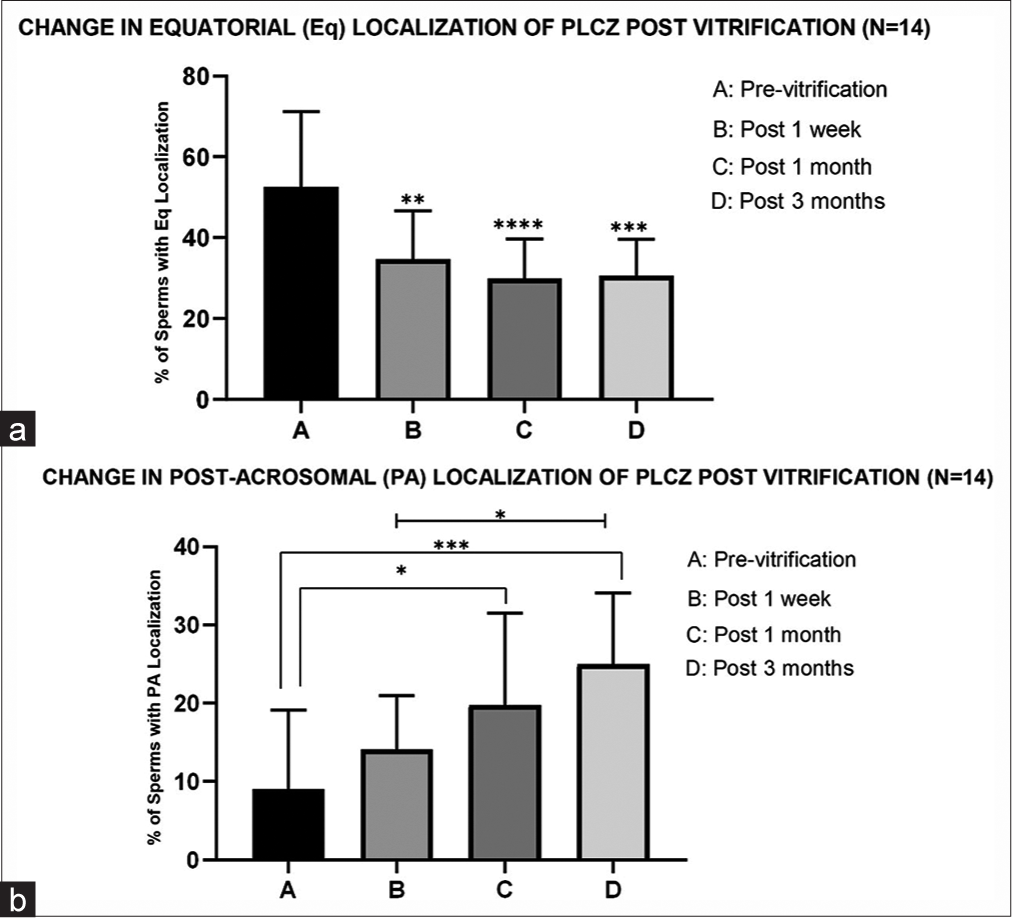
- Effect of vitrification on phospholipase C zeta (PLC ζ) localization (n = 14). (a) The graph indicates a significant change (decrease) in PLC ζ equatorial localization post 1 week, 1 month, and 3 months of vitrification compared to the pre-vitrification status. *** indicates P < 0.001, **indicates P < 0.01. (b) In this graph“***” and “*” indicate a significant increase (P ≤ 0.001 and P ≤ 0.05) in PLC ζ post-acrosomal localization post 3 months of vitrification compared to the pre-vitrification and post-1-week status, respectively. “*” indicates a statistically significant increase in PLC ζ post-acrosomal localization post 1 month of vitrification compared to the pre-vitrification status (P ≤ 0.05).
DISCUSSION
Fourteen infertile men were recruited in the present study to decipher the temporal effects of vitrification on sperm vitality, motility, morphology, and PLC ζ expression. There was no significant difference in sperm parameters between normal and subnormal samples pre-vitrification (P ≥ 0.05).
In post-vitrified samples, there was a consistent reduction in the total number of spermatozoa with normal morphology, motility, vitality, and PLC ζ expression. However, not all such decreases were found to be statistically significant. The detrimental effect was most prominently evident in the 1st week of storage with a steep fall in all parameters under study. This may be accounted for varied mechanisms of cell damage during cell freezing such as liquid phase transition changes, increased lipid peroxidation, toxicity due to osmotic stress during saturation and dilution of the freezing medium, and oxidative stress. Interestingly however, it was noted that sample aliquots on 3 months of analysis showed slightly improved parameters such as motility, vitality, morphology, and PLC ζ expression, although the change was not found statistically significant for any parameter. Nonetheless, the consistent improvement seen for all sperm parameters, on 3rd month of analysis, is compelling enough to reflect if vitrification, its own uniqueness is more effective in maintaining sperm quality for longer time periods, as compared to other cryopreservation methods which mostly report either no significant effect or detrimental effects at different storage time points.[25]
Among different parameters measured, sperm vitality and motility were found to be significantly affected by the vitrification process. Spermatozoa are considered motile and viable when they have integrity of sperm membranes. When semen is cryopreserved, sperms are exposed to a cold shock, formation of ice crystals, and cellular dehydration, which result in irreversible damage. The known disadvantages of vitrification are adverse changes in the composition of membrane lipid which causes increasing membrane damage, inducing acrosome reaction and apoptosis.[26] Thus, the ultra-fast freezing method through vitrification may have significantly reduced sperm motility and viability parameters.
Motility is one of the most important factors affecting sperm quality.[26] The results are in accordance with most of the previous cryopreservation studies where the average difference in the motility of cryopreserved and thawed spermatozoa is reported to fall by approximately 50% of the motility before freezing.[27] However, considerable inter-individual fluctuation does occur as seen in the present study. As far as motility decrease depending on sample type is concerned, the overall decrease in 3 months in the present study was higher in subnormal ejaculates by 5% than in normal ejaculates (32% vs. 37%). It was also found that the change in motility of subnormal samples was significantly greater than in the normal samples at all time points (P ≤ 0.01; P ≤ 0.05; and P ≤ 0.01 for 1 week, 1 month, and 3 months). This could be since already compromised sperms in subnormal samples are more prone to cryoinjuries than their normal counterparts.[28]
Motility is said to be partially dependent on mitochondrial function. The decrease in motility of the spermatozoa has thus been attributed to damage to the mitochondrial membrane which has also been reported post-cryopreservation.[29] The ATP generated by oxidative phosphorylation in the inner mitochondrial membrane is transferred to the microtubules, to drive motility. Therefore, an impairment of mitochondrial activity may explain the reduction in motility.
Similar to the finding of this study, several previous studies investigating the effect of cryopreservation on the sperm membrane have found a decline in the membrane integrity after thawing leading to low sperm survival rates.[30] Interestingly, it was noted that in most cryopreserved specimens, the values of vitality were oddly lower than those of post-thaw motility; when theoretically, the former should be always higher than the latter. Several factors are involved in this apparently paradoxical phenomenon. First, it should be noted that the compromise of membrane integrity may occur by damage to the sperm head membrane, tail membrane, or both. More specifically, cryodamage to the head and tail membrane may occur independently; the presence of a damaged head membrane does not necessarily indicate the damage of tail membrane.[31] Since the eosin dye exclusion method only checks the intactness of the head membrane, it may consider sperms with partially damaged head membrane but intact tail membranes (and motile) as dead cells and consequently lead to bias. In addition to that, it is known that during the freezing-thawing process, disruption of sperm head membrane can occur more easily than the tail and a compromised head membrane but intact tail membrane is also the main transitional state of membrane cryodamage.[31] Besides these, other factors could also aggravate the membrane permeability: the presence of glycerol promoting eosin permeation into slightly damaged sperm, increased sperm permeation due to sperm preparation techniques, huge osmotic difference imposed on dilution of a hyperosmotic medium of cryoprotective sperm among others, due to which sperm with as light membrane damage might also have been stained with eosin. Hence, rather than employing a test that only checks a particular membrane function such as that by the eosin test, it seems favorable to use a more rigorous test that can identify all types of membrane integrity simultaneously for cryopreserved samples. One such test called the HOS-EY test (eosin Y exclusion and hypo-osmotic swelling test) has been suggested for such use.[31]
In the present study, a subtle decrease in the normal morphological forms of sperms was also seen post-vitrification. Tail defects, particularly coiling and sharp bends, were the most common finding. Head defects, including vacuolated heads and megalo-heads, were also often noted in samples post-warming. Similar findings have been reported in a few previous studies.[20] Nonetheless, in the present study, none of the decrease in the normal forms at all time points were found to be statistically significant. Hence, compared to change in other parameters, morphology was the least affected parameter under study. The findings suggest that vitrification may be less physically damaging to sperms given that the mechanical injuries due to ice crystal formation are avoided in the vitrification process. The finding is supported by other studies.[32]
Both the presence and localization pattern of PLC ζ on spermatozoa were found to be decreased and altered after the vitrification-devitrification process, although the decrease was not statistically significant. PLC ζ is a soluble cytosolic protein that could be lost during the cryopreservation process secondary to the loss of sperm membrane integrity by events such as ice crystal formation during the warming phase, setting of hypertonic conditions during the freezing phase, cell dehydration, osmotic shock, or the generation of ROS species leading to PLC ζ membrane leakage.[33] PLC ζ expression may also change due to acrosomal-like reactions that occur during the cryopreservation process and swelling of the subacrosomal space due to detachment between the internal acrosomal membrane and the nuclear membrane.[16] In addition to this, PLC ζ can simply undergo denaturation due to pH and temperature changes.[11]
Kashir et al., in 2011, found a significant reduction (20– 56%) in PLC ζ immunofluorescence levels in spermatozoa following cryopreservation in six sperm donors.[34] Heytens et al., in 2009, have compared the quantitative PLC ζ expression before and after cryopreservation using Western blot whereby they showed a reduced intensity of the band representing the full-length PLC ζ protein after thawing (relative intensity 0.30 ± 0.03 in frozen samples versus 1.00 ± 0.27 in the fresh ones, P < 0.01).[35] Similarly, a more recent study by Moreau et al., 2019 also reported a significant decrease in the percentage of spermatozoa exhibiting PLC ζ post-cryopreservation (44% ± 22% vs. 34 ± 19%, P < 0.05).[36]
However, it is important to stress that none of the previous studies employed vitrification as their sperm cryopreservation method. Thus, the insignificant decrease in PLC ζ at all checked time points as reported in our study proposes the hypothesis that the vitrification method of cryopreservation of sperms may have a protective effect upon the PLC ζ content of sperm as compared to the conventional methods of freezing sperms. Although the better approach will be to compare both techniques together in a single study.
Interestingly, the changes with vitrification in PLC ζ-positive sperm were found to be significantly correlated with change in morphology at all time points. This result suggests that sperm morphology may predict the presence of PLC ζ, and thus, the selection of sperms with normal morphology after cryopreservation may be a smart choice for further ART procedures downstream. Similar findings are also reported in other studies.[37] The finding also indicates that sperm cryopreservation procedures that have a protective effect on morphology, such as vitrification, may also have a similar effect on PLC ζ and, thus, the oocyte activation potential of the sperm.
On immunofluorescence analysis, five prominent localizations on the sperm head were evident: acrosomal (A); post-acrosomal (PA); equatorial (E); acrosomal and equatorial (A+E); post-acrosomal and equatorial (PA+E). However, the predominant pattern of localization was an equatorial region, both pre- and post-storage (54.69% in the fresh sample, 36.65% in 1st week, 30.50% in 1st month, and 30.98% in 3 months). This is in accordance with the study finding that identified the equatorial position as the predominant pattern in human sperms, and the study of Yoon and Fissore, in bull sperms.[24,38] However, on post-vitrification and warming, there was a marked reduction in the dominance of equatorial localization (P ≤ 0.001) and the sperm head showed an indefinite punctate distribution of the PLC ζ protein. Nonetheless, the equatorial and increasing post-acrosomal localizations could still be discerned in most of the samples. The increasing dominance of post-acrosomal localization showed a significant increase (P ≤ 0.01 and P ≤ 0.05) post 3 months of vitrification compared to the previtrification and post-1-week status, respectively.
This localization in particular may be an advantageous attribute of the vitrification process since the post-acrosomal localization of PLC ζ has been reported to have the highest positive correlation with oocyte fertilization.[23] However, this is in contrast to the finding of Moreau et al. which reported the effect of slow manual freezing on the percentage of spermatozoa exhibiting PLC ζ at the post-acrosomal position significantly decreased after thawing (8% vs. 5%, p≤0.05).[36] The vitrification technique thus may affect PLC ζ localization in different ways.
CONCLUSIONS
At present, conventional sperm freezing is still the primary method used for sperm cryopreservation at ART clinics, but sperm vitrification has shown great advantages. Implications for quality maintenance for long storage periods, insignificant changes in the number of sperms with normal morphology and PLC ζ expression, and the increase in post-acrosomal localization of PLC ζ post-vitrification evident in this study, suggest that this method of cryopreservation in particular might help conserve the PLC ζ localization pattern in sperms implicated for successful fertilization. Thus, the vitrification method of sperm cryopreservation may be the method of choice for routine clinical use in ART settings.
Ethical approval
The research/study was approved by the Institutional Review Board at AIIMS, New Delhi, number IECPG-28/27.02.2020, dated August 31, 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Human sperm cryopreservation: Update on techniques, effect on DNA integrity, and implications for ART. Adv Urol. 2012;2012:854837.
- [CrossRef] [PubMed] [Google Scholar]
- Novel approaches to the cryopreservation of human spermatozoa: History and development of the spermatozoa vitrification technology. J Reprod Biotechnol Fertil. 2011;2:128-45.
- [CrossRef] [Google Scholar]
- Semen freezing past, present and future. 2017. Available from https://indianfertilitysociety.org/pdf/nexus_4_final_29.01.17_8.15pm.pdf [Last accessed on 2021 Jan 27]
- [Google Scholar]
- Thermodynamic aspects of vitrification. Cryobiology. 2010;60:11-22.
- [CrossRef] [PubMed] [Google Scholar]
- Principles of cryopreservation by vitrification. Methods Mol Biol. 2015;1257:21-82.
- [CrossRef] [PubMed] [Google Scholar]
- Cryopreservation of testicular and epididymal sperm: Techniques and clinical outcomes of assisted conception. Clinics (Sao Paulo) 2013(1 Suppl):131-40.
- [CrossRef] [PubMed] [Google Scholar]
- Cryopreservation of embryos and oocytes in human assisted reproduction. Biomed Res Int. 2014;2014:307268.
- [CrossRef] [PubMed] [Google Scholar]
- Process and pitfalls of sperm cryopreservation. J Clin Med. 2017;6:89.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrastructural changes in membranes and acrosome of human sperm during cryopreservation. Arch Androl. 1990;25:29-40.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrastructural alterations of frozen-thawed Asian elephant (Elephas maximus) spermatozoa. Int J Androl. 2006;29:346-52.
- [CrossRef] [PubMed] [Google Scholar]
- Implication of apoptosis in sperm cryoinjury. Reprod Biomed Online. 2010;21:456-62.
- [CrossRef] [PubMed] [Google Scholar]
- Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod Biomed Online. 2018;37:327-39.
- [CrossRef] [PubMed] [Google Scholar]
- Proteomic characteristics of human sperm cryopreservation. Proteomics. 2014;14:298-310.
- [CrossRef] [PubMed] [Google Scholar]
- Sperm cryopreservation: Principles and biology. J Infertil Reprod Biol. 2020;8:43-8.
- [CrossRef] [Google Scholar]
- Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17-8.
- [CrossRef] [PubMed] [Google Scholar]
- The outcome of ART in males with impaired spermatogenesis. J Hum Reprod Sci. 2008;1:73-6.
- [CrossRef] [PubMed] [Google Scholar]
- Aetiology of failed and abnormal fertilization after intracytoplasmic sperm injection. Hum Reprod. 1995;10:2623-9.
- [CrossRef] [PubMed] [Google Scholar]
- A preliminary study of human sperm citrate synthase expression in patients with failed ICSI cycles. J Res Biol. 2020;10:1-11.
- [Google Scholar]
- Oocyte metaphase arrest and release: Triggers and pathways. J Infertil Reprod Biol. 2021;9:59-64.
- [Google Scholar]
- Basics of human andrology. (1st ed). Singapore: Springer Nature; 2017. p. :1-536.
- [CrossRef] [Google Scholar]
- PLCζ: A sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development. 2002;129:3533-44.
- [CrossRef] [PubMed] [Google Scholar]
- Total levels and proportions of sperm exhibiting phospholipase C ζ (PLC ζ) are significantly correlated with fertilization rates following intracytoplasmic sperm injection. Fertil Steril. 2015;104:561-8.
- [CrossRef] [PubMed] [Google Scholar]
- The pattern of localization of the putative oocyte activation factor, phospholipase Cz, in uncapacitated, capacitated, and ionophore-treated human spermatozoa. Hum Reprod. 2008;23:2513-22.
- [CrossRef] [PubMed] [Google Scholar]
- Does storage time influence postthaw survival and pregnancy outcome? An analysis of 11,768 cryopreserved human embryos. Fertil Steril. 2010;93:109-15.
- [CrossRef] [PubMed] [Google Scholar]
- Vitrification is not superior to rapid freezing of normozoospermic spermatozoa: Effects on sperm parameters, DNA fragmentation and hyaluronan binding. Reprod Biomed Online. 2014;28:352-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cryopreservation of human semen and prepared sperm: Effects on motility parameters and DNA integrity. Fertil Steril. 2001;76:892-900.
- [CrossRef] [PubMed] [Google Scholar]
- Recent advances in boar semen cryopreservation. Soc Reprod Fertil Suppl. 2009;66:51-66.
- [Google Scholar]
- A new system of sperm cryopreservation: Evaluation of survival, motility, DNA oxidation, and mitochondrial activity. Andrology. 2019;7:293-301.
- [CrossRef] [PubMed] [Google Scholar]
- Detrimental effects of cryopreservation on the structural and functional integrity of the sperm membrane. Arch Androl. 1991;27:155-60.
- [CrossRef] [PubMed] [Google Scholar]
- Cryodamage to plasma membrane integrity in head and tail regions of human sperm. Asian J Androl. 2000;2:135-8.
- [Google Scholar]
- Cryopreservation of human spermatozoa by vitrification versus conventional rapid freezing: Effects on motility, viability, morphology and cellular defects. Eur J Obstet Gynecol Reprod Biol. 2019;234:14-20.
- [CrossRef] [PubMed] [Google Scholar]
- Controlled ice nucleation in cryopreservation--a review. Cryobiology. 2013;66:85-92.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of cryopreservation and density-gradient washing on phospholipase C ζ concentrations in human spermatozoa. Reprod Biomed Online. 2011;23:263-7.
- [CrossRef] [PubMed] [Google Scholar]
- Reduced amounts and abnormal forms of phospholipase C ζ (PLCζ) in spermatozoa from infertile men. Hum Reprod. 2009;24:2417-28.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of phospholipase PLC ζ in human spermatozoa: impact of cryopreservation. Andrology. 2019;7:315-8.
- [CrossRef] [PubMed] [Google Scholar]
- Electrical activation of oocytes after intracytoplasmic sperm injection: A controlled randomized study. Fertil Steril. 2009;91:133-9.
- [CrossRef] [PubMed] [Google Scholar]
- Release of phospholipase C zeta and (Ca2+) I oscillation inducing activity during mammalian fertilization. Reproduction. 2007;134:695-704.
- [CrossRef] [PubMed] [Google Scholar]






