Translate this page into:
Immunohistochemical expression of programmed death ligand (PD-L1) is associated with poor differentiation, higher tumor-infiltrating lymphocytes, and higher t and n stage in colorectal adenocarcinoma: A single-center analytical study of 52 cases in South India
*Corresponding author: Dr. Ramya Katta, MD (pathology), Assistant Professor, 203, Sai Nilaya, Nelson Mandela Park Road, LIC colony, Vijayawada drkattaramya@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Katta R, Reddy TR, Sai PD, Padma M. Immunohistochemical expression of programmed death ligand (PD-L1) is associated with poor differentiation, higher tumor-infiltrating lymphocytes, and higher t and n stage in colorectal adenocarcinoma: A single-center analytical study of 52 cases in South India. J Lab Physicians. 2024;16:164-9. doi: 10.1055/s-0043-1771019
Abstract
Objectives:
Colorectal adenocarcinoma is the fourth leading cause of cancer-related mortality worldwide. These tumors are heterogeneous in terms of genomic alterations, immune response of the microenvironment, drug responsiveness, and biological behavior. Physiologically and pathologically, programmed cell death ligand 1 (PD-L1) is a key immune-regulatory molecule that suppresses immune response. PD-L1 expression on tumor cell has been implicated as a cause of immune evasion by tumor cells in many cancers. However, its activity in colorectal carcinomas is still under study. The aim of this study is to correlate PD-L1 marker expression with patient demographics, clinicopathological features, and TNM (tumor size, node involvement, and metastasis status) stage, and to find if there exists any significant correlation between them.
Materials and Methods:
The present study is a 3-year retrospective analytical study conducted in a tertiary care hospital in South India. Specimens were routinely fixed, processed, and cut. Hematoxylin and eosin–stained sections and corresponding PD-L1-stained sections were analyzed and data were tabulated.
Statistical analysis:
Results were tabulated and statistical analysis was done using Statistical Package for the Social Sciences (SPSS) software (version 24). The chi-squared test was used to calculate the value of significance (p value).
Results:
PD-L1 expression on tumor cells was significantly associated with female gender, right-sided tumors, poorly differentiated tumors, higher number of tumor-infiltrating lymphocytes, and higher T and N statuses.
Conclusion:
High PD-L1 expression on tumor cells is a marker for poor prognosis. A subset of colorectal adenocarcinoma may benefit with anti-PD-L1-targeted therapy.
Keywords
immunotherapy
mismatch repair
TNM staging
Tumor-infiltrating lymphocytes
INTRODUCTION
Programmed death ligand (PD-L1) is an imperative molecule for physiological immune tolerance by “self ” cells and pathological immune evasion by tumor cells.[1,2] PD-L1, when expressed by cells, downregulates immune responses by binding to either programmed death-1 (PD-1/CD279) or CD80 (B7-1) on T cells or antigen presenting cells (APCs),[3] resulting in reduced cytotoxic T-cell response by inhibiting T-cell proliferation and cytokine production.[4] PD-L1 expression on tumor cells results in immune evasion by tumor cells and has been associated with poor prognosis and reduced disease-free survival in many malignancies, like melanoma,[5] esophageal, gastric, hepatocellular, and urothelial carcinomas.[6] The role of PD-L1 expression in colorectal carcinoma is less clear, and has conflicting results about disease-free survival and prognosis.[7–10]
With the above considerations, the present study was taken up to identify if there exists any relationship between PD-L1 expression on colorectal tumor cells and/or immune cells and age, site, gender, histological variants, mucinous differentiation, number of tumor-infiltrating lymphocytes(TIL), presence of peritumoral lymphoid aggregates, and TNM (tumor size, node involvement, and metastasis status) staging.
MATERIALS AND METHODS
The present study is a 3-year retrospective analytical study conducted in the department of pathology of a tertiary care teaching center in South India. Institutional ethical clearance was taken as per the departmental protocol. All the colorectal resection specimens that were received and diagnosed as colorectal adenocarcinomas between March 2019 and March 2022 were included in the study. Cases that were diagnosed as nonepithelial malignancies, re-exploration surgery specimens, and small biopsy samples were excluded from the study. Clinical features, demographic facts, and other relevant data were retrieved from the departmental archives.
Specimens were routinely fixed using 10% buffered for-malin and representative tissue bits were processed using automatic tissue processor (Leica TP1020). Sections stained with hematoxylin and eosin stain were used to identify the histological variant, differentiation, mucinous differentiation, medullary morphology, calculate the number of TIL, presence or absence of peritumoral lymphoid aggregates, depth of invasion, and nodal status. Grossly tumor location was considered as left when it was present distal to the splenic flexure.
Hematoxylin and eosin–stained tumor sections were examined under 400X magnification in Olympus 21x microscope for assessing the number of tumor-infiltrating lymphocytes and calculating peritumoral lymphoid aggregates (PTLA). A semiquantitative scale was used for scoring. TIL scores were given based on the percentage of tumor cells with overlying lymphocytes (0 = none or rare; 1+ = 5%; 2+ = ≥5% and <25%; and 3+= ≥25%). PTLA were scored between 0 and 2 (0 = none; 1 = a few, often <5; and 2 = > 5).
Immunohistochemistry for PD-L1 (clone: 22C3, Agilent, Dako) was performed manually using the manufacturer’s recommendations and protocol. Antigen retrieval was performed using heat-induced epitope retrieval by means of a pressurized decloaker. PD-L1 expression on tumor cells and immune cells was assessed using a four-tier system[12] (score 0: <1% of cells show staining, score 1: 1 to <5% of cells show staining, score 2: 5 to <10% of cells show staining, and score 3: 10% or more cells show staining; Figure 1). In the present study, for tumor cells only linear membranous staining of any intensity was considered positive, while cytoplasmic staining was considered negative. For assessing the staining in immune cells, different types of immune cells (macrophages, dendritic cells, or lymphocytes), infiltrating the tumor, intra and peritumoral stroma were considered. Staining of any intensity and any location (punctate or granular) was considered positive.[13]
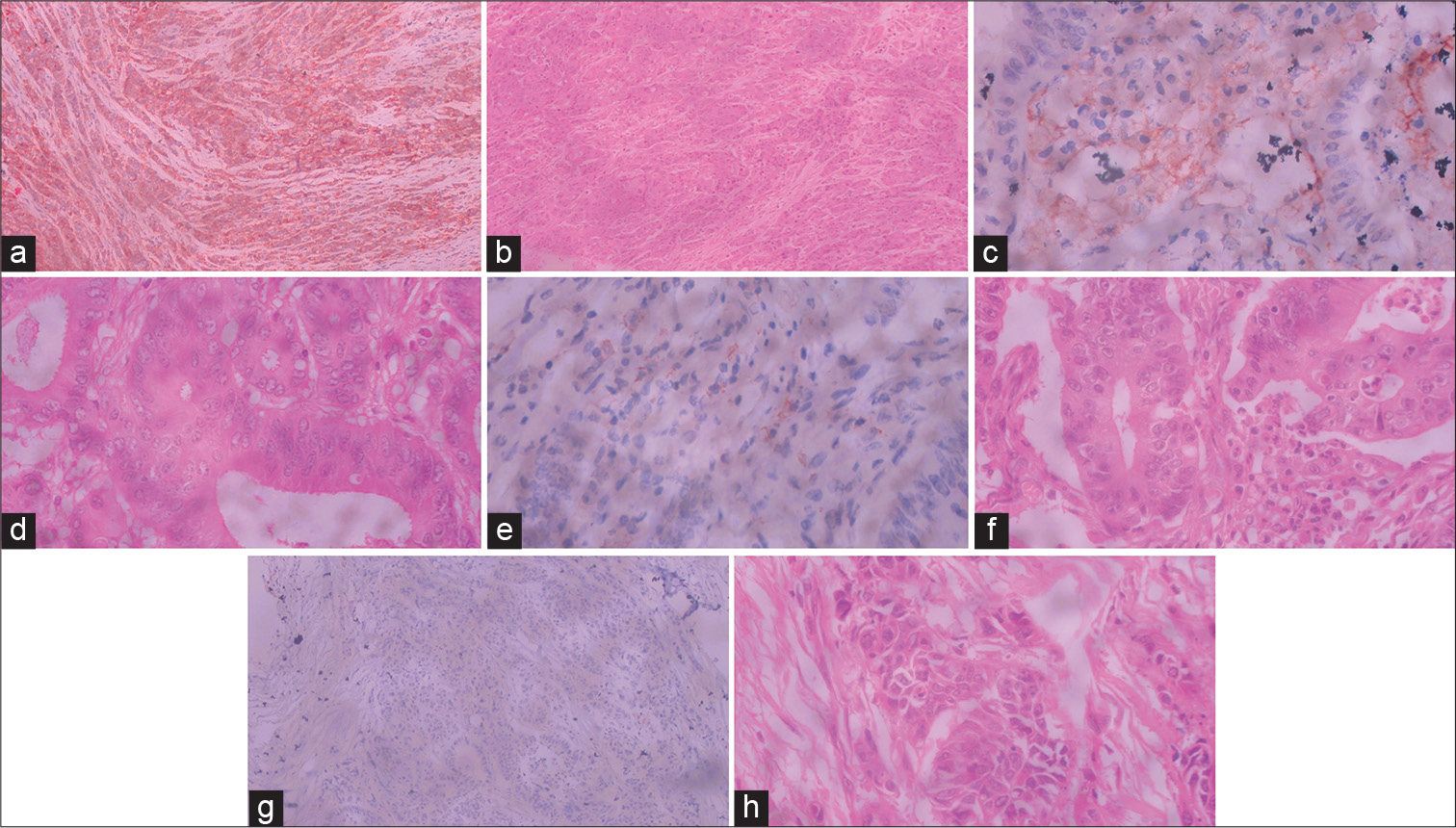
- Representative photomicrographs of the four-tier scoring system for PD-L1 expression on neoplastic cells in colorectal carcinoma. (a). Photomicrograph showing tumor cells with diffuse and strong membranous positivity for PD-L1 in more than 10% cells - score 3 (10X magnification). (b) Corresponding hematoxylin and eosin–stained section (10X magnification). (c) Photomicrograph showing tumor cells with membranous positivity for PD-L1 in 5 to 10% cells - score 2 (40X magnification). (d) Corresponding Hematoxylin and eosin–stained section (40X magnification). 1e). Photomicrograph showing tumor cells with membranous positivity for PD-L1 in 1 to 5% cells - score 1 (40X magnification). (f) Corresponding Hematoxylin and eosin–stained section (40X magnification). (g). Photomicrograph showing tumor cells with membranous positivity for PD-L1 in less than 1% cells - score 0 (10X magnification). (h) Corresponding hematoxylin and eosin–stained section (40X magnification).
Results were tabulated in Microsoft Excel 2020 version and statistical analysis was done using Statistical Package for the Social Sciences (SPSS) software (version 24). The chi squared test was used to calculate value of significance (p value). A p value ≤0.05 was considered significant.
RESULTS
After considering all the inclusion and exclusion criteria, a total of 52 cases were included in the present analysis.
Patient demographics and tumor histopathological features: The mean age of the population in the present study was 56 years (range: 34–77 years). The male-to-female incidence ratio was 0.52 with a slight female preponderance. The most common location for the tumor was distal to splenic flexure of the colon. Of the 52 cases included, most cases showed well to moderate differentiation (42 cases). Lymphovascular invasion was seen in 19 cases and perineural invasion was seen in 14 cases. The most common stages were T3 (22 cases) and N0 (27 cases) status.
PD-L1 expression on neoplastic cells: Of the 52 tumors included in the present study, 34 cases showed immunohistochemical expression of PD-L1 marker on neoplastic cells (65.3%). The highest score of 3 was seen only in eight cases (15.3% cases). PD-L1 expression was significantly associated with female gender, right-sided tumors, poorly differentiated tumors, tumors with mucinous differentiation, tumors with medullary morphology, higher number of tumor-infiltrating lymphocytes, and higher T and N statuses. However, there was no significant correlation with age, presence of peritumoral lymphocytes, lymphovascular invasion, or perineural invasion (Table 1).
| Clinicopathological parameters | N | PD-L1 expression in neoplastic cells | P value | |||
|---|---|---|---|---|---|---|
| Score 0 | Score 1 | Score 2 | Score 3 | |||
| Age | 0.187 | |||||
| <60 y | 33 | 13 | 4 | 9 | 7 | |
| ≤60 y | 19 | 5 | 5 | 8 | 1 | |
| Gender | 0.014 | |||||
| Females | 34 | 7 | 7 | 12 | 8 | |
| Males | 18 | 11 | 2 | 5 | 0 | |
| Tumor laterality | 0.041 | |||||
| Left | 30 | 11 | 6 | 12 | 1 | |
| Right | 22 | 7 | 3 | 5 | 7 | |
| Tumor differentiation | 0.003 | |||||
| Well | 24 | 13 | 6 | 4 | 1 | |
| Moderate | 18 | 3 | 1 | 11 | 3 | |
| Poor | 10 | 2 | 2 | 2 | 4 | |
| Mucinous differentiation | 0.014 | |||||
| ≤50% | 19 | 2 | 6 | 8 | 3 | |
| < 50% | 33 | 16 | 3 | 9 | 5 | |
| Medullary morphology | 0.041 | |||||
| Present | 20 | 3 | 4 | 10 | 3 | |
| Absent | 32 | 15 | 5 | 7 | 5 | |
| Number of tumor-infiltrating lymphocytes | 0.003 | |||||
| 0 | 8 | 6 | 0 | 2 | 0 | |
| 1 | 12 | 3 | 1 | 8 | 0 | |
| 2 | 18 | 6 | 6 | 4 | 2 | |
| 3 | 14 | 3 | 2 | 3 | 6 | |
| Peritumoral lymphoid aggregates | 0.083 | |||||
| 0 | 32 | 15 | 6 | 9 | 2 | |
| 1 | 16 | 3 | 3 | 6 | 4 | |
| 2 | 4 | 0 | 0 | 2 | 2 | |
| Perineural invasion | 0.212 | |||||
| Absent | 38 | 12 | 5 | 14 | 7 | |
| Present | 14 | 6 | 4 | 3 | 1 | |
| Lymphovascular invasion | 0.187 | |||||
| Absent | 33 | 13 | 4 | 9 | 7 | |
| Present | 19 | 5 | 5 | 8 | 1 | |
| Depth of invasion | 0.008 | |||||
| Tumor invades submucosa (T1) | 2 | 1 | 1 | 0 | 0 | |
| Tumor invades muscularis propria (T2) | 19 | 10 | 6 | 3 | 0 | |
| Tumor invades subserosa or nonperitonealized pericolic tissue (T3) | 22 | 5 | 0 | 12 | 5 | |
| Tumor invades other organs or perforates visceral peritoneum (T4) | 9 | 2 | 2 | 2 | 3 | |
| Nodal involvement | 0.0001 | |||||
| No nodal involvement (N0) | 27 | 15 | 3 | 9 | 0 | |
| 1–3 regional lymph node involvement (N1) | 23 | 3 | 4 | 8 | 8 | |
| 4 or more regional nodal involvement (N2) | 2 | 0 | 2 | 0 | 0 | |
PD-L1 expression on tumor-infiltrating mononuclear cells: In the present study, only 11 of 52 cases showed immunohistochemical expression of PD-L1 marker on tumor-infiltrating mononuclear cells (21.1%). PD-L1 expression on immune cells was significantly higher in the cases that had higher tumor-infiltrating lymphocytes and cases with a higher N status. All the other parameters studied showed no significant correlation, as depicted in Table 2.
| Clinicopathological parameters | N | PD-L1 expression in immune cells | p value | ||
|---|---|---|---|---|---|
| Score 0 | Score 1 | Score 2 | |||
| Age | 0.779 | ||||
| < 60 y | 33 | 27 | 5 | 1 | |
| ≤60 y | 19 | 14 | 4 | 1 | |
| Gender | 0.896 | ||||
| Females | 34 | 27 | 6 | 1 | |
| Males | 18 | 14 | 3 | 1 | |
| Tumor laterality | |||||
| Left | 30 | 24 | 4 | 2 | 0.345 |
| Right | 22 | 17 | 5 | 0 | |
| Tumor differentiation | |||||
| Well | 24 | 18 | 5 | 1 | 0.614 |
| Moderate | 18 | 16 | 2 | 0 | |
| Poor | 10 | 7 | 2 | 1 | |
| Number of tumor-infiltrating lymphocytes | 0.041 | ||||
| 0 | 8 | 7 | 0 | 1 | |
| 1 | 12 | 12 | 0 | 0 | |
| 2 | 18 | 10 | 7 | 1 | |
| 3 | 14 | 12 | 2 | 0 | |
| Peritumoral lymphoid aggregates | 0.836 | ||||
| 0 | 32 | 25 | 6 | 1 | |
| 1 | 16 | 12 | 3 | 1 | |
| 2 | 4 | 4 | 0 | 0 | |
| Depth of invasion | 0.405 | ||||
| Tumor invades Submucosa (T1) | 2 | 1 | 1 | 0 | |
| Tumor invades Muscularis propria (T2) | 19 | 13 | 4 | 2 | |
| Tumor invades sub serosa or non peritonealized pericolic tissue (T3) | 22 | 20 | 2 | 0 | |
| Tumor invades other organs or perforates visceral peritoneum (T4) | 9 | 7 | 2 | 0 | |
| Nodal involvement | 0.008 | ||||
| No nodal involvement (N0) | 27 | 22 | 4 | 1 | |
| 1–3 regional lymph node involvement (N1) | 23 | 19 | 4 | 0 | |
| 4 or more regional nodal involvement (N2) | 2 | 0 | 1 | 1 | |
DISCUSSION
PD1/PD-L1 axis is a well-deciphered immune check point that has been implicated in creating an immunosuppressive tumor microenvironment that helps the tumor cells in escaping from immune-mediated destruction. In most tumors, tumor cells upregulate the expression of PD-L1 by either of the two mechanisms: first, being innate immune resistance and, second, being adaptive immune resistance.[11] Innate immune resistance is seen most commonly in tumor of the lung where tumor cells upregulate the expression of PD-L1 due to constitutive activation of oncogenic signals (ALK or EGFR mutations)[14]. However, in colorectal carcinoma, PD-L1 expression upregulation seems to be more of an adaptive immune resistance as an immune response to local inflammatory mediators and tumor-infiltrating immune cells.[11,15]
Only 5 of 34 (14.7%) cases that showed PD-L1 expression in neoplastic cells showed diffuse positivity, while the remaining cases showed focal positivity. In addition, it was found that the cases that showed focal positivity expressed PD-L1 mostly along tumor–stromal interface. Our findings are similar to those obtained by Lee et al.[16]
In the present study, we found that 65.3% of cases included showed positivity of PD-L1 on neoplastic cells. The positivity rate for PD-L1 in neoplastic cells shows high variation between various studies (5–89%).[12,16,17]
This high variability could be due to use of different immunohistochemical clones for testing, different assay conditions,[18] different scoring methods,[16,18] different cutoff values,[16] tissue microarrays, focal and heterogenous pattern of expression of the marker,[17] and selection bias[19] as most studies were retrospective and single centered.[20] A previous study by Hirsch et al[21] showed that the concordance rate of positivity in lung cancers using different PD-L1 clones was about 50% only. Thus, standardization of PD-L1 staining is essential for initiation of anti-PD-L1 immunotherapy in colorectal carcinomas.
In the present study, a significant association was found between tumor cells expression of PD-L1 and female gender, right-sided lesions, tumors with mucinous differentiation, tumors with medullary morphology, high number of tumor infiltrating lymphocytes, poor differentiation, and higher T and N statuses. Our findings are in correlation with the findings of various other studies.[11,12,17] Many authors also compared PD-L1 expression on neoplastic cells with microsatellite instability and found that there was a significant association between them.[16,22] Inaguma et al[17] in their study established that PD-L1 expression on tumor cells was associated with “stem cell-like” phenotype and BRAF mutation.
In the current analysis, a significant association was found between the expression of PD-L1 on immune cells and higher number of tumor-infiltrating lymphocytes and higher nodal status. Our findings are similar to that observed by Valentini et al.[20] Most studies claim that the function of PD-L1 expression on immune cells is still puzzling. Thompson et al[23] state that the expression of PD-L1 on immune cells was associated with poor prognosis in renal carcinoma patients and good prognosis in urothelial carcinoma patients, while Koganemaru et al[19] found that high PD-L1 expression on immune cells was associated with good prognosis in stage III colorectal carcinomas.
In general, the results of various clinical trials investigating use of immunotherapy in colorectal carcinoma patients have mostly been unsatisfactory.[11] However, a specific sub set of patients, those with high microsatellite instability (MSI-H) or mismatch repair protein deficiency (dMMR), seem to have been benefited.[13,22,24] Various studies showed that colorectal carcinomas arising in patient with MSI-H or dMMR have a high tumor mutational burden with the formation of many neoantigens, which act as targets for the host’s antitumor immune response. Upregulation of the expression of PD-L1, CTLA-4, LAG-3, or other immune checkpoints by neoplastic cells or immune cells can act as an escape mechanism. It is hypothesized that PD-L1 positivity on neoplastic cells might be a predictive marker for such cases, which may benefit with add-on immunotherapy.[11,20] Lee et al[16] indicated that PD-L1 expression in dMMR colorectal carcinomas may be used to identify cases with poorer prognosis despite having high level of tumor-infiltrating lymphocytes.
Limitation of the present study is that PD-L1 expression was not compared with the molecular profile of the tumor and clinical outcomes of the patients. Furthermore, being a single-center study, a selection bias may have been seen.
CONCLUSIONS
In conclusion, in the present study, it was established that high PD-L1 expression on tumor cells was associated with poorer prognosis as it is associated with poorly differentiated tumors and tumors with higher T and N stages.
Compliance with Ethical Principles
This study was approved by the institutional review board (GMC/IEC/001/2022, Registration No. ECR/467/Inst/AP/2013/RR19), and the study conforms to the ethical norms and standards in the Declaration of Helsinki.
Conflict of Interest
None declared.
Funding
None.
References
- Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069-1086.
- [CrossRef] [PubMed] [Google Scholar]
- PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10:727-742.
- [Google Scholar]
- PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- [CrossRef] [PubMed] [Google Scholar]
- Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739-745.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757-1766.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L1 and survival in solid tumors: a meta-analysis. PLoS One. 2015;10:e0131403.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49:2233-2242.
- [CrossRef] [PubMed] [Google Scholar]
- The vigorous immune microenvi-ronment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43-51.
- [CrossRef] [PubMed] [Google Scholar]
- B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS One. 2013;8:e76012.
- [CrossRef] [PubMed] [Google Scholar]
- Stromal PD-1/PD-L1 expres-sion predicts outcome in colon cancer patients. Clin Colorectal Cancer. 2019;18:e20-e38.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medul-lary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol. 2016;29:1104-1112.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical impact of PD-L1 expression for survival in curatively resected colon cancer. Cancer Invest. 2020;38:406-414.
- [CrossRef] [PubMed] [Google Scholar]
- Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: a guide to immunohistochemistry implementation and interpretation. Pathology. 2021;53:141-156.
- [CrossRef] [PubMed] [Google Scholar]
- Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355-1363.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48-61.
- [CrossRef] [PubMed] [Google Scholar]
- Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol. 2016;29:1433-1442.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathological profile, immunophenotype and geno-type of CD274 positive colorectal carcinomas. Mod Pathol. 2017;30:278-285.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15:55.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of programmed death-ligand 1 expression in patients with stage III colorectal cancer. Cancer Sci. 2017;108:853-858.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L1 expression in colorectal cancer defines three subsets of tumor immune microenvironments. Oncotarget. 2018;9:8584-8596.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12:208-222.
- [CrossRef] [PubMed] [Google Scholar]
- PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509-2520.
- [CrossRef] [PubMed] [Google Scholar]
- Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174-17179.
- [CrossRef] [PubMed] [Google Scholar]
- Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182-1191.
- [CrossRef] [PubMed] [Google Scholar]






