Translate this page into:
Changing prevalence and antibiotic drug resistance pattern of pathogens seen in community-acquired pediatric urinary tract infections at a tertiary care hospital of North India
Address for correspondence: Dr. Sarman Singh, Division of Clinical Microbiology and Molecular Medicine, Department of Laboratory Medicine, All India Institute of Medical Sciences, New Delhi - 110 029, India. E-mail: sarman_singh@yahoo.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
AIMS AND OBJECTIVES:
The aim and objective of this study was to assess the temporal changes in the microbiological profiles and antimicrobial resistance patterns of uropathogens in pediatric community-acquired UTI.
MATERIALS AND METHODS:
This is a retrospective analysis of data collected over a Scattered period of 5 years. The baseline data collected were from January to December 2009, and the second period considered for comparison was from January to December 2014. Urine specimens from children (<17 years) suspected of UTI were cultured by a semi-quantitative method on cysteine lactose electrolyte-deficient medium. Antibiotic sensitivity was put up by Kirby–Bauer disc diffusion method as per the Clinical and Laboratory Standard Institute guidelines.
RESULTS:
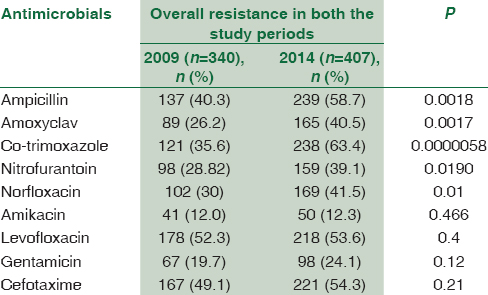
In the year 2009, 340 of 2104 (16.15%) urine specimens yielded significant colony count, whereas in 2014, it was 407 of 2212 (18.39%) (P = 0.051). Escherichia coli was the predominant pathogen and was significantly more prevalent in girls than in boys (P < 0.0001) during both periods. There was a significant overall increase in resistance to ampicillin (from 40.29% to 58.72%), amoxyclav (from 26.17% to 40.54%), nitrofurantoin (from 28.82% to 39.06%), and norfloxacin (from 30% to 41.42%). However, the maximum increase in the resistance was noted for co-trimoxazole from 35.58% in 2009 to 63.39% in 2014 (P = 0.0000058). The prevalence of extended-spectrum beta-lactamases (ESBLs) has also significantly increased from 21.7% to 33.16% (P = 0.0045).
CONCLUSION:
Although E. coli remains the prime pathogen in pediatric UTI, the prevalence of resistance has dramatically increased over the 5-year study period. Our study highlights the emergence of community-acquired ESBL-producing uropathogens in children proclaiming treatment challenges.
Keywords
Antimicrobials
children
extended-spectrum beta-lactamase producers
microbiological profile
resistance pattern
urinary tract infection
Introduction
In pediatric population, urinary tract infection (UTI) is considered to be one of the most prevailing bacterial infections.[1] Over the recent decades, the importance of UTI has been increasingly recognized in children. Moreover, the occurrence of UTI during childhood may have acute and/or chronic consequences, such as impaired renal function, renal scarring, and hypertension.[2] Thus, timely and effective management of UTI with appropriate antibiotic administration is of immense importance to reduce the risk of long-term consequences in children.[3] Although Gram-negative enteric bacilli, particularly Escherichia coli and Klebsiella spp., are the leading pathogens, Enterococcus and Staphylococcus aureus are also emerging as prominent agents in the recent years.[4] The increasing trend in the resistance pattern of bacterial pathogens against commonly used antimicrobials is a cause of concern in the recent years.[5] The diagnosis of UTI may be missed in infants and young children, as urinary symptoms are minimal and often nonspecific. Therapy for these children requires urine culture and appropriate antimicrobial sensitivity testing. E. coli, in particular, is increasingly recognized as the most notorious uropathogen with substantial antimicrobial resistance worldwide. Furthermore, the first choice of an antimicrobial agent for empiric treatment of pediatric UTI is not well established.[6] The Indian Society of Paediatric Nephrology recommends the use of oral cephalosporin, amoxyclav, ciprofloxacin, and ofloxacin in uncomplicated UTI in children depending on the local sensitivity pattern.[7]
However, the resistance pattern of uropathogens, particularly in children, has not been extensively studied in India.[8] Recent studies on pediatric UTI concerning microbiological profile and antibiotic susceptibility are limited in India. Moreover, there is a paucity of recent literature regarding any temporal change in the spectrum of uropathogens and their antimicrobial resistance profile which is of great importance in deciding the current treatment options and to note any changes in resistance patterns. The present study was carried out to determine the microbiological and antimicrobial resistance profiles of all consecutive pediatric UTI patients, referred for urine culture and sensitivity testing from pediatric outpatient department of our tertiary care institute during two different periods 5 years apart, i.e., January–December 2009 and January–December 2014. In addition, the temporal changes in the antimicrobial resistance profiles over this 5-year period were also studied in detail.
Materials and Methods
Study design and patient population
This is a retrospective observational study in which medical laboratory data were reviewed. The study was conducted in the division of clinical microbiology and molecular medicine of a tertiary care hospital in India over two different time periods, i.e. January–December 2009 and January–December 2014. During each period, the patient population comprised children <17 years consecutively presenting to the pediatric outpatient clinic of our institute with symptoms suggestive of UTI. The exclusion criteria were hospitalization, presence of catheters, and children who were already on antibiotic treatment. A total of 2104 children were included during the first study period (January–December 2009), of which 1288 (61.2%) were males and 816 (38.8%) were females. Whereas, during the second study period (January–December 2014), 2212 children were included, of which 1320 (59.6%) were males and 892 (40.32%) were females.
Microbiological methods
Urine culture was performed using a semi-quantitative technique on cysteine lactose electrolyte-deficient medium (CLED agar, Hi-Media, India).[9] Urine was cultured using a calibrated bacteriological 4 mm loop (0.05 ml) on CLED agar, and colonies were counted after overnight incubation at 37°C. For boys and girls, 104 and 105 CFU/ml of bacterial growth of a single type was considered as significant bacteriuria, respectively. Whereas, any number of colonies from a suprapubic aspirate were considered significant.[10] Isolates thus obtained in the culture were further identified on the basis of Gram-stain, motility test, and routine biochemical reactions. Antibiotic sensitivity was put up by Kirby–Bauer disc diffusion method on Mueller–Hinton agar (MHA) (Hi-Media India) adhering to the Clinical Laboratory Standards Institute (CLSI) guidelines.[11]
Extended-spectrum beta-lactamase detection
This test was performed on MHA by disc diffusion method as recommended by the CLSI. A ≥5 mm increase in zone diameter for ceftazidime (30 μg) tested in combination with clavulanate versus its zone diameter when tested alone confirmed an extended-spectrum beta-lactamase (ESBL)-producing organism.[12]
Statistical analysis
The statistical analyses were performed using Statistical Package for Social Science software (SPSS version 20.0, IBM Corp., Armonk, NY, USA) and Microsoft office Excel 2010. Chi-square test was used for categorical variables. P < 0.05 was considered statistically significant.
Results
A total of 2104 urine samples from equal number of children were included during the first study period (January–December 2009), of which 1288 (61.2%) were males and 816 (38.8%) were females (males: females ratio – 1.58:1). Of these, 340 urine specimens (16.2%) yielded significant growth. Whereas, during the second study period, 2212 children were included, of which 1320 (59.6%) were males and 892 (40.3%) were females (male: female ratio – 1.48:1). The age range was from 0.1 to 17 years in both periods. Of these, 407 (18.3%) specimens showed significant bacteriuria on semi-quantitative culture. There was a marginal increase in the prevalence of UTI from 16.2% (during the first study period) to 18.3% (during the second study period). This difference was statistically significant (P = 0.051). Prevalence of UTI in male children was 18.0% and 13.2% in the respective periods (P = 0.08), whereas among female children, they were 13.2% and 22.4%, respectively (P = 0.00001) [Table 1].

Bacteriology
The microbiological profile in relation to gender is shown in Table 2. Gram-negative bacteria were the most common uropathogens isolated during both periods. E. coli predominated during both the study periods and was isolated from a total of 194 (57.1%) children in the first and in in the second period, i.e. 226 (55.5%). E. coli was significantly more prevalent in girls (74.1% during the first study period and in 61.5% during the second study period) than in boys (49.2% and 49.7% in respective study periods) (P < 0.0001). Klebsiella sp. was found to be second in frequency.

Antimicrobial resistance
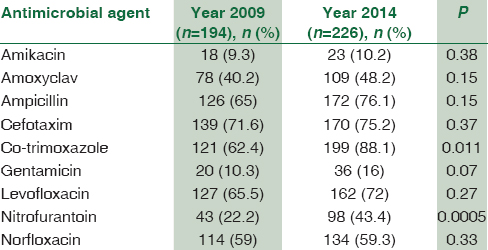
The overall resistance to frequently used antibacterial agents is shown in Table 3. The results revealed that there was a statistically significant increase in the resistance prevalence of isolated uropathogens to some tested antibiotics from 2009 to 2014 except for gentamicin and cefotaxime. The resistance was found to be highest for oral drugs than that for injectable antimicrobial agents. There was a significant overall increase in the resistance to ampicillin (from 40.2% to 58.7%), amoxyclav (from 26.2% to 40.5%), nitrofurantoin (from 28.8% to 39.0%), and norfloxacin (from 30% to 41.4%). However, the maximum increase in the resistance was noted for co-trimoxazole from 35.5% in 2009 to 63.3% in 2014 (P = 0.0000058). In addition, there was a significant difference in antimicrobial resistance pattern of E. coli when compared between the two study periods [Table 4].
The prevalence of ESBL has significantly increased from 21.7% to 33.2% over a period of 5 years from 2009 to 2014 (P = 0.0045). Moreover, the prevalence of ESBL-producing pathogen was more in females than that in males during both the study periods (P < 0.05). When the resistance pattern to tested antimicrobials was analyzed gender wise, we observed that there was no significant difference.
Discussion
Uropathogens are showing a rising trend in the antimicrobial resistance pattern throughout the world. The uplift of the resistant strains can be attributed to either over the counter availability of the drugs accounting for the misuse of many antimicrobials or by empirical treatment. This problem is especially important in the developing countries as majority of patients often cannot afford to consult a physician or have a laboratory analysis made. Our hospital being an apex tertiary care institute caters to a large number of patients from North India. The present study was undertaken to analyze the change in microbiological and antimicrobial resistance profile of uropathogens isolated from children with UTI over a period of 5 years (from 2009 to 2015). Overall UTI prevalence was found to be 16.2% and 18.3% during the respective periods (P = 0.051). Thus, there was a marginal increase in the overall prevalence of UTI, which did not account for any statistical significance. However, when the data were analyzed among the females over the 5-year study period, we found that there was a significant increase in the prevalence of UTI from 13.2% in 2009 to 22.4% in 2014 (P = 0.00001). However, being a hospital-based study, the exact prevalence cannot be extrapolated to pediatric population in general, for which a population-based study is required.
In concordance with other studies, E. coli was the most common etiological agent causing UTI in pediatric population in both males and females and during both the study periods.[13] Moreover, it was significantly prevalent in girls as compared to boys during both the study periods (P < 0.0001). Our results are in congruence with previously published studies, which showed that E. coli was more prevalent in girls.[14] This difference could be attributed to the differences in the preputial flora in males and females.[15]
There was a significant increase in the prevalence of overall resistance over a 5-year period. As expected, resistance was found to be highest for commonly prescribed oral drugs than that of injectable antimicrobial agents during both the study periods. In addition, there was a significant rise in resistance to ampicillin, amoxyclav, nitrofurantoin, co-trimoxazole, and norfloxacin. The highest increase in resistance was found against co-trimoxazole over the 5-year period which is one of the most common and empirically prescribed drugs in children. The drug is also available on the counters and many parents give this drug on their own without consulting the doctors. The highest increase in resistance to co-trimoxazole over a period signifies over-the-counter availability and misuse of this drug. Various other studies worldwide have also demonstrated a rising trend in the resistance, especially to trimethoprim, with or without sulphonamide, which has been the first choice for treatment of children with UTI for a long time.[1516]
There has been a tremendous increase in ESBL prevalence as well over a 5-year period. Moreover, it was found to be significantly higher in females than in males during both the study periods (P < 0.05). ESBLs are enzymes that mediate resistance to the beta-lactam antibiotics, including extended-spectrum cephalosporins and monobactams.[17] Overall, the isolation of ESBL-producing organisms typically occurs in hospital settings and other health-care facilities; however, such organisms have begun to disseminate in the community, and the incidence of community-onset UTIs due to ESBL-producing strains has increased worldwide.[1819] The rising trend of ESBL producers in pediatric UTI is quite alarming as it limits the choices and outcome of antimicrobial treatment and should be dealt seriously.
The growing evidence from the literature points to the necessity to follow resistance rates over a time period. With increasing resistance and limited treatment options, such measures will be even more important. Although antimicrobial resistance evolution is not entirely new, the high-level emergence of ESBL-producing uropathogens in children and that too in community-acquired UTI is definitely alarming. In the light of our report, the high antimicrobial resistance among uropathogens affecting pediatric age group needs to be addressed and reviewed seriously. It is noteworthy that almost all isolated organisms in our study were found to have increased resistance to most routine antibiotics that were tested. Therefore, a strict antibiotic prescription policy needs to be accentuated to prevent the future threat of notoriously resistant uropathogens and limited antimicrobial options.
Conclusion
The prevalence of antimicrobial resistance has dramatically increased over the study period. Our report highlights the evolution of ESBL-producing uropathogens in children in community-acquired UTI. This alarming uplift in antimicrobial resistance warrants future challenges in the successful treatment of pediatric UTI.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Incidence rate of first-time symptomatic urinary tract infection in children under 6 years of age. Acta Paediatr. 1998;87:549-52.
- [Google Scholar]
- Day- and night-time blood pressure elevation in children with higher grades of renal scarring. J Pediatr. 2003;142:117-22.
- [Google Scholar]
- Oral versus initial intravenous therapy for urinary tract infections in young febrile children. Pediatrics. 1999;104(1 Pt 1):79-86.
- [Google Scholar]
- Factors affecting renal scarring in posterior urethral valves. J Pediatr Urol. 2006;2:569-74.
- [Google Scholar]
- Antimicrobial resistance in community and nosocomial Escherichia coli urinary tract isolates, London 2005-2006. Ann Clin Microbiol Antimicrob. 2008;7:13.
- [Google Scholar]
- Choice of antibiotic for empirical therapy of acute cystitis in a setting of high antimicrobial resistance. Indian J Med Sci. 2006;60:53-8.
- [Google Scholar]
- Revised statement on management of urinary tract infections. Indian Pediatr. 2011;48:709-17.
- [Google Scholar]
- Antibiotic resistance pattern in uropathogens. Indian J Med Microbiol. 2002;20:96-8.
- [Google Scholar]
- Mackie and McCartney Practical Medical Microbiology (14th ed). New York: Churchill Livingstone Publishers, Longman; 2003.
- Performance Standards for Antimicrobial Susceptibility Testing: 17th Informational Supplement, CLSI M100-S17. Vol Vol. 27. Wayne, PA: Clinical and Laboratory Standards Institute; 2007.
- 2005 Guidelines by CLSI/NCCLS-CLSI Informational Supplement. In: Approved Standard M100-S15. Wayne, PA: Clinical and Laboratory Standards Institute; 2000.
- [Google Scholar]
- Antibiotic resistance patterns of pediatric community-acquired urinary infections. Braz J Infect Dis. 2008;12:321-3.
- [Google Scholar]
- First urinary tract infection in neonates, infants and young children: A comparative study. Pediatr Nephrol. 2006;21:1131-7.
- [Google Scholar]
- Febrile urinary tract infection in children: Ampicillin and trimethoprim insufficient as empirical mono-therapy. Pediatr Nephrol. 2008;23:597-602.
- [Google Scholar]
- Antibiotic resistance patterns of outpatient pediatric urinary tract infections. J Urol. 2013;190:222-7.
- [Google Scholar]
- Risk factors in community-acquired urinary tract infections caused by ESBL-producing bacteria in children. Pediatr Nephrol. 2010;25:919-25.
- [Google Scholar]
- Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum beta-lactamases. J Antimicrob Chemother. 2006;57:780-3.
- [Google Scholar]
- Predictors of mortality from community-onset bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2008;29:671-4.
- [Google Scholar]





