Translate this page into:
Correlation of Surrogate Markers of Insulin Resistance with Fasting Insulin in Type 2 Diabetes Mellitus Patients: A Study of Malwa Population in Punjab, India
Address for correspondence: Navneet Kaur, MSc, Department of Biochemistry, Guru Gobind Singh Medical College and Hospital, Faridkot 151203, Punjab, India (e-mail: navmann23@yahoo.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context
Insulin resistance (IR) and abnormal insulin secretion play a key role for the development of type 2 diabetes mellitus (DM).
Aims
We investigated the surrogate markers of IR, i.e., Homeostasis Model Assessment (HOMA), Quantitative Insulin Sensitivity Check Index (QUICKI), McAuley, and Fasting Insulin Resistance Index (FIRI) in type 2 DM patients. Also, fasting insulin (FI) levels were estimated in type 2 diabetics. Further, the correlation of FI with other surrogate markers of IR in type 2 DM was done.
Settings and Design
A hundred newly diagnosed patients with type 2 DM from Malwa population, Punjab, were considered for evaluation. Another 100 healthy individuals (age and sex-matched) were examined as controls.
Methods and Material
Fasting blood glucose, FI, and lipid profile were estimated, and IR was calculated using McAuley index (McA), HOMA, QUICKI, and FIRI.
Statistical Analysis Used
The statistical analysis was performed on the above-mentioned clinical interpretations. The Cohen's kappa test was used to affirm the agreement.
Results
FI levels in patients with type 2 diabetes were significantly higher (20.8 ± 9.05 µIU/L) than controls (7.93 ± 1.01 µIU/L). IR by surrogate markers was found significant in the study group. The 76% patients with type 2 diabetes ended up as resistant to insulin by FI measurement, almost equivalent to McA, 80%; HOMA, 88%; FIRI, 88%; and QUICKI, 90%. A notable correlation was highlighted between FI and McA manifesting IR (p < 0.01, r = −0.85). We calculated the statistical correlation of FI with HOMA, QUICKI, and FIRI indices (p < 0.01, r = 0.93; p < 0.01 r = −0.92; and p < 0.01, r = +0.93, respectively). The agreement visible from Cohen's kappa test also affirms the same (k = 0.9 for McA).
Conclusion
We concluded that all the surrogate markers for IR were specific when compared with FI, but in terms of sensitivity McA was found to be more sensitive as it includes markers of dyslipidemia, which is the precipitating factor of metabolic derangements so as the IR in type 2 DM.
Keywords
serum insulin
FIRI
HOMA index
insulin resistance
McAuley index
QUICKI
type 2 diabetes mellitus
Key Message
The statistical analysis and Cohen's kappa agreement affirm that the McA is sensitive as well as specific to fasting insulin for the insulin resistance evaluation in the Malwa population of Punjab.
Introduction
Diabetes mellitus (DM), popular as “diabetes”, is a condition where a person has elevated blood glucose levels, as the body cells in such persons do not absorb the glucose. This hyperglycemia leads to vascular and other complications.[1] Type 2 DM is one of the most prevalent types of diabetes, roughly comprising 90% of the diabetic population across any country. It is higher in developed than in developing countries. According to a survey conducted in 2018, around 500 million global population was diagnosed as type 2 diabetic.[2] Type 2 DM is a heterogeneous group of disorders with a complex etiology that develops in response to genetic and environmental influences. Insulin resistance (IR) and abnormal insulin secretion are key to the development of type 2 DM. Despite the controversy concerning the primary defect, most of the existing studies embrace the perspective that IR leads up to insulin secretory defects.[3]
IR is one of the common risk markers for type 2 DM.[4] Studies revealed that β-cell destruction had already occurred before the patient gets diagnosed with impaired fasting blood glucose levels.[1] Therefore, IR assessment is very important in the management of DM. The Minimal Model Approximation of the Metabolism of Glucose (MMAMG) is considered as a gold standard method for measuring IR. Other techniques are the euglycemic insulin clamp method and intravenous glucose tolerance test. But these methods are impractical and difficult when adopted in population-based studies.[5,6] Several indirect methods have also been proposed for the estimation of IR, out of which Homeostasis Model Assessment (HOMA),[7] Quantitative Insulin Sensitivity Check Index (QUICKI),[8] McAuley index (McA),[9] and Fasting Insulin Resistance Index (FIRI)[10] are widely adopted. Unfortunately, IR is not calculated clinically, excluding the research settings. An enhanced level of fasting blood glucose is the initial indicator of IR. On the contrary, serum fasting insulin (FI) levels are increased much before this actually occurs. The early detection and control are very important to prevent hyperinsulinemia and its associated complications.[11]
FI has been found as an accurate marker for diagnosing IR in the normoglycemic population.[12] So, the quantification of FI can be adopted as a generic and viable diagnostic marker in contrast to the other indirect techniques used for the diagnosis of IR. So, this study was initiated to determine the role of IR in type 2 diabetics. Here, FI levels were used as a measure of IR. Moreover, the results were compared among each other with the IR value quantified through indirect methods like, McAuley, HOMA, QUICKI, and FIRI.
Subjects and Methods
The materials and methods used in the present study are discussed in the subsequent subsections.
Study Population
Keeping in view of the availability and feasibility of the participants, a nonrandom convenient sampling technique was adopted. So, 100 freshly diagnosed type 2 DM patients from Malwa population of Punjab, India who attended the Department of Medicine at Guru Gobind Singh Medical College and Hospital, Faridkot, Punjab, India were considered in our study. Alongside, 100 healthy individuals (age and sex-matched) were taken as controls.
Selection Criteria
Informed written consent was obtained from study population including controls. The clinical history of the patients (including age, sex, drug, smoking, alcohol consumption, etc.) was obtained from them. According to ADA criteria, patients having fasting plasma glucose ≥ 126 mg% (7 mmol/L) and 2-hour OGTT post-load glucose load ≥ 200 mg% were included in this study.[13] Patients suffering from any disease of thyroid, liver, kidney or heart failure, stroke, and those having the previous history of insulin treatment were excluded from the study. Pregnant and lactating women were also excluded.
At baseline, in the morning, we sampled the venous blood for the estimation of the blood glucose, triglycerides, total and HDL cholesterol, and FI after a sufficient 10-hour overnight fasting period. Now, the blood glucose was calculated using the glucose oxidase approach. The serum total cholesterol, HDL cholesterol, and triglycerides have been calculated by using standard enzymatic spectrophotometric approaches. The serum LDL cholesterol has been estimated with the Friedewald equation,[14] except in the case where the triglycerides were more than 400 mg/dL. Special investigations such as serum FI and IR were calculated using various methods. FI was estimated using Accu-Bind Insulin ELISA Kit, a solid phase enzyme-linked immunosorbent assay, that works on the concept of immobilized sandwich.[15] The variation coefficient for inter- and intraassay was 6.29 and 7.67%, respectively and the sensitivity of assay was 1.5 µIU/L.
Data Analysis
IR was measured using indirect methods HOMA,[7] QUICKI,[8] McA,[9] and FIRI.[10] The following equations were used to calculate IR using these methods.[7-10]
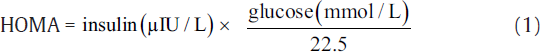

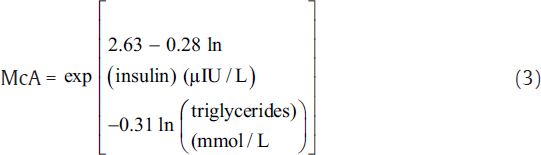
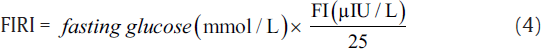
The aforementioned indices were contrasted with FI to investigate the sensitivity and specificity for the prediction of IR. Thereafter, we perform the statistical analysis using the statistical package for social sciences compatible for Windows version 12.0. Alongside, the descriptive analysis was also performed which comprised the calculation of mean values and standard deviations for the continuous variables. Also, the categorical or unequivocal variables were differentiated using the chi-square test. We consider that the p-values less than 0.05 are said to be statistically relevant or significant. Correlation was also measured among surrogate markers of IR. Finally, the agreement was measured using Cohen's kappa test to further support the obtained results.[16]
Results
The results obtained on the basis of considered parameters or markers are presented in the subsequent subsections.
Determinants of IR among Type 2 Diabetics
Here, the FI levels were significantly higher in the study group, i.e., type 2 diabetic patients (20.8 ± 9.05 μIU/L) as compared with control subjects (7.92 ± 1.01 μIU/L). Here, the patients were considered insulin resistant only if FI ≥ 12 μIU/L.[17] IR estimated by HOMA, McA, QUICKI, and FIRI techniques was found to be significant in the type 2 DM group. Here, the mean ± SD values for McAuley, HOMA, QUICKI, and FIRI approaches were estimated as 5.02 ± 1.23, 8.15 ± 3.92, 0.29 ± 0.02, and 7.33 ± 3.53, respectively. We considered the cut-off threshold for a person to be insulin resistant for the aforementioned measures as, McA ≤ 5.8, HOMA ≥ 2.6, QUICKI ≤ 0.33, and FIRI ≥ 2.52 (considering normal reference values of concerned parameters).[17]►Table 1 presents the baseline characteristics of type 2 diabetics and control groups.
| Characteristics | Type 2 DM group | Control group | p-Value | Significance |
|---|---|---|---|---|
| Age (years) | 44.6 ± 10.97 | 45.74 ± 8.2 | ≥ 0.05 | NSa |
| Total cholesterol (mg/dL) | 227.7 ± 16.6 | 162.0 ± 15.2 | ≤ 0.05 | Sb |
| Triglycerides (mg/dL) | 186.19 ± 66.2 | 87.16 ± 21.6 | ≤ 0.001 | HSc |
| HDL cholesterol (mg/dL) | 40.01 ± 6.01 | 44.6 ± 6.09 | ≤ 0.05 | Sb |
| LDL cholesterol (mg/dL) | 153.19 ± 6.5 | 99.4 ± 11.7 | ≤ 0.001 | HSc |
| Fasting blood glucose (mg/dL) | 155.8 ± 24.3 | 73.8 ± 8.6 | ≤ 0.05 | Sb |
| Fasting insulin (μIU/L) | 20.8 ± 9.05 | 7.92 ± 1.01 | ≤ 0.001 | HSc |
| McAuley index | 5.02 ± 1.23 | 7.95 ± 0.93 | ≤ 0.001 | HSc |
| HOMA index | 8.15 ± 3.92 | 1.42 ± 0.35 | ≤ 0.001 | HSc |
| QUICKI index | 0.29 ± 0.02 | 1.68 ± 0.13 | ≤ 0.001 | HSc |
| FIRI | 7.33 ± 3.53 | 1.04 ± 0.36 | ≤ 0.001 | HSc |
Abbreviations: DM, diabetes mellitus; FIRI, Fasting Insulin Resistance Index; HDL, high-density lipoprotein; HOMA, Homeostasis Model Assessment; LDL, low-density lipoprotein; QUICKI, Quantitative Insulin Sensitivity Check Index.
aNS, nonsignificant, i.e., p-value was ≥ 0.05.
bS, significant, i.e., p-value was ≤ 0.05.
cHS, highly significant, i.e., p-value was ≤ 0.001.
In our study group, a significant negative correlation was witnessed among FI and McA in expressing the IR (p < 0.01, r = −0.85). ►Fig. 1A depicts the correlation of serum insulin levels with IR by McAuley. Moreover, FI is proved to have a statistically relevant correlation with HOMA, QUICKI, and FIRI measures (p < 0.01, r = 0.93; p < 0.01, r = −0.92; p < 0.01, r = +0.93, respectively). ►Fig. 1B depicts the correlation of serum insulin levels with IR by HOMA and ►Fig. 1C presents the correlation of serum insulin levels with IR by QUICKI. Lastly, the correlation of serum insulin levels with IR by FIRI is shown in ►Fig. 1D. The FI evaluation in type 2 DM patients estimated IR in 76% patients, quite alike to other practices (McA, 80%; HOMA, 88%; QUICKI, 90%, and FIRI, 88%).

- Correlation plots: (A) Correlation of FI levels with IR by McA, (B) correlation of FI levels with IR by HOMA, (C) correlation of FI levels with IR by QUICKI, (D) correlation of FI levels with IR by FIRI. FI, fasting insulin; FIRI, Fasting Insulin Resistance Index; HOMA, Homeostasis Model Assessment; IR, insulin resistance; QUICKI, Quantitative Insulin Sensitivity Check Index.
Prevalence of IR in Different Age Groups
IR calculated by FI in this study group was grouped according to age. We found out that the age group of 41 to 50 year-old patients contributed the maximum number of diabetic as well as insulin-resistant patients. On the other hand, patients of more than 60 years of age were the least contributor.
Prevalence of IR
Sensitivity of IR by FI was calculated comparing it with other surrogate markers of IR. Out of type 2 diabetics detected as insulin resistant by McA, 95% of them were found insulin resistant by FI method. This means that only 5% of these patients were not detected as insulin resistant by FI. Similarly, among the type 2 diabetics detected as insulin resistant using HOMA and FIRI indices, almost 86.4% were detected as insulin resistant when tested using FI method. This indicates that 13.6% type 2 diabetics detected as insulin resistant using HOMA and FIRI were not insulin resistant when FI test was performed. Also, among all the patients detected by QUICKI, 84.4% were detected as insulin resistant and further 15.6% type 2 diabetics were not found as insulin resistant when compared with FI test. ►Fig. 2(A–D) shows the pie charts for IR sensitivity as depicted by different surrogate markers.

- Sensitivity plots. (A) McA, (B) HOMA, (C) QUICKI, and (D) FIRI. FIRI, Fasting Insulin Resistance Index; HOMA, Homeostasis Model Assessment; McA, McAuley index; QUICKI, Quantitative Insulin Sensitivity Check Index.
►Table 2 depict the different metrics (including sensitivity, specificity, accuracy, and Kappa agreement) of FI when compared with surrogate markers. Even the comparison of accuracy for the conducted estimation using four surrogate markers is presented in Fig. 3A. This figure clearly shows that the accuracy for McA is higher in contrast to other surrogate markers. To further support the above results, we conducted Cohen’ kappa test to compute the agreement. The agreement values obtained using Cohen's kappa test are also depicted in Fig. 3B. The kappa agreement values also illustrate that there is substantial agreement between FI and McA. In contrast, a moderate agreement is witnessed for HOMA, FIRI, and QUICKI.

- Comparative plots: (A) Accuracy, and (B) comparison by Chen's Kappa agreement.
| Methods/Parameters | TP | FP | TN | FN | Sensitivity (%) | Specificity (%) | Accuracy (%) | kappa |
|---|---|---|---|---|---|---|---|---|
| McA | 76 | 0 | 20 | 4 | 95 | 100 | 95.56 | 0.90 |
| HOMA | 76 | 0 | 12 | 12 | 86 | 100 | 88 | 0.603 |
| FIR | 76 | 0 | 12 | 12 | 86 | 100 | 88 | 0.603 |
| QUICKI | 76 | 0 | 10 | 14 | 84 | 100 | 86 | 0.521 |
Abbreviations: FIRI, Fasting Insulin Resistance Index; FN, false negative; FP, false positive; HOMA, Homeostasis Model Assessment; McA, McAuley index; QUICKI, Quantitative Insulin Sensitivity Check Index; TN, true negative; TP, true positive.
Discussion
In the present study, the relevance of FI as a reliable method for the detection of IR in contrast to the conventional methods such as McAuley, HOMA, QUICKI, and FIRI indices has been assessed. For this reason, recently diagnosed type 2 DM patients were considered to correlate McA, HOMA, QUICKI, and FIRI with FI for IR assessment. Hypertension and hyperinsulinemia occur as a consequence of IR.[18] Generally, the IR can be estimated using various techniques. But, most of these techniques are not easy to deploy for the clinical practice. As the compensatory hyperinsulinemia is significantly correlated with IR, so it can render more viable way to detect insulin-resistant type 2 diabetics instead of measuring glucose intolerance.[19]
McA is considered as one of the most accurate indirect methods employed to detect IR and more specific as well as sensitive when contrasted with the output depicted by the MMAMG process. Moreover, the specificity as well as the sensitivity of this detection came out to be more for McA.[9] In the past, it has been discovered that FI test is an accurate marker in normoglycemic population[20] and the current study demonstrated that the FI test can detect the IR in a diabetic patient in similar significance to McA. The estimation of HOMA-IR shows enhanced sensitivity and specificity for IR specifically for children and adolescents. So, it is demonstrated as a more robust approach than QUICKI.[21] Another study conducted by Huguette et al[22] demonstrated the prevalence of IR by HOMA-IR and QUICKI as 53.9 and 55.7%, respectively.
Rutter et al[23] also demonstrated a relevant correlation for FI and HOMA-IR. Similarly, the results depicted by Conwell et al[24] manifested a significant correlation between HOMA-IR and QUICKI, along with sensitivity (p < 0.01). They concluded that HOMA-IR, QUICKI, and FI have depicted a strong correlation with sensitivity in obese children and adolescents. In another study,[25] all these measures (McA, HOMA, and QUICKI) came out to be highly significant in the diabetic group, and also these parameters correlated well with insulin levels (p < 0.01). The FI test showed notable levels of specificity and sensitivity in contrast to McA, QUICKI, and HOMA measures.[26] In another study, Gates et al[27] found FIRI to be significantly correlated with parameters associated with the metabolic syndrome. Moreover, Rudvik, and Månsson[28] recommended HOMA-IR, QUICKI, and FIRI for IR analysis in clinical studies. All the three parameters had significant correlation with gold standard euglycemic clamp.
In our present study, we have found that 86.36% patients who proved to be insulin-resistant using HOMA and FIRI indices were also insulin-resistant by FI test. This indicates that 13.6% of patients detected as insulin-resistant using HOMA and FIRI practices were missed while adopting the FI test. Also, among the people mentioned as insulin resistant by QUICKI, only 84.4% patients were detected by FI. The primary reason for this indication is supported through the limitations associated with HOMA, QUICKI, and FIRI in the existing studies. HOMA and FIRI are computed using a formula based on fasting glucose and FI therefore it will reflect hepatic insulin sensitivity.[29] Another study[30] also supported these outcomes by analyzing the composite insulin resistance which comprises of hepatic as well as peripheral resistance to evaluate the insulin sensitivity in diabetics.
In the present study, the FI test was found to be well correlated with McA, QUICKI, HOMA, and FIRI (p < 0.005). McA was found to be more sensitive marker when compared with FI. The outcomes for the evaluation of IR as depicted through conventional approaches and FI were almost similar. Moreover, the viability of McA was evaluated using Cohen's kappa test, which illustrated acceptable and adequate consensus.
Conclusion
We concluded that all the surrogate markers for IR were specific when compared with FI, but in terms of sensitivity McA was found to be more sensitive as it includes markers of dyslipidemia, which is the precipitating factor of metabolic derangements so as the IR in type 2 DM.
Ethical Approval
The approval from the Ethical Committee of the Guru Gobind Singh Medical College and Hospital, Faridkot, Punjab, India, was taken before conducting the present study.
Source(s) of Financial Support
None.
Acknowledgment
The authors are thankful to the patients and volunteers involved in the present study to provide their consent. They are also thankful to the clinical staff of Guru Gobind Singh Medical College and Hospital, Faridkot, Punjab, India, for their support during the study.
Conflict of Interest
None declared.
Funding
No internal or external funding is related to this work.
References
- Diabetes treatment—bridging the divide. N Engl J Med. 2007;356(15):1499-1501.
- [CrossRef] [PubMed] [Google Scholar]
- Global prevalence of type 2 diabetes over the next ten years. . 2018;67(Suppl. 01):1.
- [CrossRef] [Google Scholar]
- Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(03):173-194.
- [CrossRef] [PubMed] [Google Scholar]
- How to measure insulin sensitivity. J Hypertens. 1998;16(07):895-906.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of insulin sensitivity in vivo. Endocr Rev. 1985;6(01):45-86.
- [CrossRef] [PubMed] [Google Scholar]
- Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(07):412-419.
- [CrossRef] [PubMed] [Google Scholar]
- et al Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(07):2402-2410.
- [CrossRef] [PubMed] [Google Scholar]
- et al Diagnosing insulin resistance in the general population. Diabetes Care. 2001;24(03):460-464.
- [CrossRef] [PubMed] [Google Scholar]
- A simple measure of insulin resistance. Lancet. 1995;346(8967):120-121.
- [CrossRef] [PubMed] [Google Scholar]
- Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab. 1990;71(06):1508-1518.
- [CrossRef] [PubMed] [Google Scholar]
- Obesity, independent of insulin resistance, is a major determinant of blood pressure in normoglycemic Hong Kong Chinese. Metabolism. 2000;49(12):1523-1528.
- [CrossRef] [PubMed] [Google Scholar]
- Classification and diagnosis of diabetes-2020. Diabetes Care. 2020;43(01):14-31.
- [CrossRef] [PubMed] [Google Scholar]
- Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(06):499-502.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic b-cell peptides: kinetics and concentration of proinsulin, insulin and c-peptide in plasma and urine, problems with measurement methods, clinical information and literature “u overview. Clin Chem Lab Med. 1980;18:313-326.
- [CrossRef] [Google Scholar]
- A generalization of Cohen's kappa agreement measure to interval measurement and multiple raters. Educ Psychol Meas. 1988;48:921-933.
- [CrossRef] [Google Scholar]
- Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(09):1462-1470.
- [CrossRef] [PubMed] [Google Scholar]
- Fasting blood level of insulin in non-obese and non-diabetic patients with essential hypertension. Pak J Med Res. 2004;43:5-7.
- [Google Scholar]
- How good a marker is insulin level for insulin resistance? Am J Epidemiol. 1993;137(09):959-965.
- [CrossRef] [PubMed] [Google Scholar]
- Fasting insulin levels as a measure of insulin resistance in American Blacks. J Med. 2003;34(1-6):31-38.
- [Google Scholar]
- Investigating for insulin resistance and type 2 diabetes mellitus in obese children. Turk J Endocrinol Metab. 2005;1:17-22.
- [Google Scholar]
- Prevalence of insulin resistance in obese Cameroonian women. J Diab Endocrinol. 2010;1:19-26.
- [Google Scholar]
- Use of alternative thresholds defining insulin resistance to predict incident type 2 diabetes mellitus and cardiovascular disease. Circulation. 2008;117(08):1003-1009.
- [CrossRef] [PubMed] [Google Scholar]
- Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care. 2004;27(02):314-319.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of insulin resistance by indirect methods-HOMA, QUICKI, and McAuley- with fasting insulin in patients with type 2 diabetes in Gale, Sri Lanka: a Pilot Study. Outline J Health Allied Sci. 2006;5(1) Hettihewa LM, Palangasinghe S, Jayasinghe SS, Gunasekara SW, Weerarathna TP. Comparison of insulin resistance by indirect methods-HOMA, QUICKI, and McAuley- with fasting insulin in patients with type 2 diabetes in Gale, Sri Lanka: a Pilot Study. Outline J Health Allied Sci 2006;5(1):1–8
- [Google Scholar]
- Evaluation of surrogate markers for insulin resistance for defining metabolic syndrome in urban Indian adolescents. Indian pediatrics. 2014;51(04):279-284.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the FIRI (Fasting Insulin Resistance Index) and selected plasma parameters associated with insulin resistance as predictors of cardiovascular mortality in rural Chinese women. Asia Pac J Clin Nutr. 1997;6(03):200-202.
- [Google Scholar]
- Evaluation of surrogate measures of insulin sensitivity—correlation with gold standard is not enough. BMC Med Res Methodol. 2018;18(01):64.
- [CrossRef] [PubMed] [Google Scholar]
- et al Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(01):57-63.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients. The Journal of Clinical Endocrinology& Metabolism. 2004;89(09):4312-4319.
- [CrossRef] [PubMed] [Google Scholar]





