Translate this page into:
Diagnostic utility of antibodies to structural protein and fibroblast intermediate filament, vimentin, in the detection of rheumatoid arthritis
*Corresponding author: Iyyapu Krishna Mohan, Additional Professor, Department of Biochemistry, Nizam’s Institute of Medical Sciences, Panjagutta, Hyderabad, India. iyyapu@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sabitha D, Sai Baba KS, Dominic S, Rajasekhar L, Bhaskar MV, Mohammed N, et al. Diagnostic utility of antibodies to structural protein and fibroblast intermediate filament, vimentin, in the detection of rheumatoid arthritis. J Lab Physicians. 2024;16:543-51. doi: 10.25259/JLP_61_2024
Abstract
Objectives:
This study aimed to evaluate the specificity and sensitivity of serum anti-mutated citrullinated vimentin (MCV) antibodies in rheumatoid arthritis (RA) patients and to determine their diagnostic utility compared to anti-cyclic citrullinated peptide antibodies and rheumatoid factor (RF).
Materials and Methods:
This study employs a cross-sectional design. Patients were categorized using the 2010 American College of Rheumatology RA criteria. The first group consists of 60 cases, of which 30 are seropositive RA, and 30 are seronegative RA. The second group consists of 60 controls, of which 30 are RF-positive connective tissue disorder (CTD) cases and 30 are healthy. Enzyme-linked immunosorbent assay kits were used to quantify the amounts of anti-MCV and anti-cyclic citrullinated protein.
Statistical analysis:
Version 9 of GraphPad Prism was utilized for doing the statistical analysis. We employed the Kolmogorov-Smirnov test, one-way ANOVA, Student’s t-test, the Kruskal-Walli or Mann-Whitney U tests, and Fisher’s exact or Chi-Square tests for statistical analysis. To study associations between variables, Spearman’s correlation test is used.
Results:
The anti-MCV (ng/mL) median concentrations in healthy controls, CTD controls, seronegative RA, and seropositive RA are 1.487, 5.294, 10.51, and 7.985, respectively, and show a significant increase (P < 0.05) in comparison to healthy controls. Seropositive and seronegative RA subjects did not differ statistically significantly in their anti-MCV levels. According to receiver operating characteristic analysis results, anti-MCV has 100% sensitivity and 83.33% specificity in seropositive RA cases with an optimal cutoff of 6.38 ng/mL and 100% sensitivity and 83.33% specificity in RF-negative RA cases with an ideal cutoff of 6.63 ng/mL.
Conclusions:
The higher levels found in both seropositive and seronegative cases of RA suggest the possibility of using serum anti-MCV as a sensitive RA marker. Anti-MCV may help in the diagnosis of RA, especially seronegative arthritis.
Keywords
Anti-cyclic citrullinated protein
Anti-mutated citrullinated vimentin
Mixed connective tissue disorder
Rheumatoid arthritis
Rheumatoid factor
INTRODUCTION
The most prevalent systemic immunoinflammatory rheumatic disease is rheumatoid arthritis (RA), with a frequency of <1% globally.[1] Its diagnosis remains primarily clinical without widely recognized diagnostic criteria. Serological tests with high titers of anti-cyclic citrullinated protein antibody (anti-CCP) and rheumatoid factor (RF) of immunoglobin M (IgM) isotype support the clinical diagnosis. However, neither their specificity nor sensitivity reaches a level that makes them reliable biomarkers for accurate diagnosis of RA. Regarding this, some researchers have reported that mutated citrullinated vimentin (MCV) antibodies are superior to both RF and anti-CCP in helping with the clinical diagnosis.[2] The present work reports the findings of MCV antibodies in RA, appropriate control groups of normal individuals, and diseases that may mimic RA.
With a 0.75% prevalence of RA in India, there are approximately seven million patients overall.[3] India’s RA prevalence is remarkably comparable to that of developed nations. The first 12 weeks following the start of early symptoms represent the ideal therapy window. However, early diagnosis is still a challenge because it relies on the patient’s physical examination, clinical history, blood investigations, and imaging analysis.[4] Blood can be used to measure anti-CCP and IgM RF in RA patients.[5] The RF test is not specific for RA even though it has a good sensitivity because it is also raised in healthy people, patients with autoimmune illnesses, chronic infections, and other rheumatic or inflammatory conditions.[6] Anti-cyclic citrullinated protein antibodies have a high specificity but a low sensitivity for early RA because cyclic citrullinated protein is not widely expressed in synovial fluid. Confirming the diagnosis of patients with a clinical diagnosis of RA can be more difficult because some of the patients have negative results for both anti-CCP and RF. At present, no biomarker is sensitive and specific enough to diagnose early seropositive and seronegative RA. Therefore, given the limitations of current RA assays, we need to evaluate a new biomarker that can identify RA patients who are seropositive or seronegative and have greater diagnostic sensitivity and specificity.
The VIM gene encodes vimentin, a type III intermediate filament with a molecular weight of 58 kDa. Vimentin is essential in wound healing and promotes excessive scarring.[7,8] Seventy-four percent of patients with RA, 14% with Sjögren’s syndrome, 2% with scleroderma, and 2% with Systemic lupus erythematosus (SLE) had mutant citrullinated vimentin antibodies (anti-MCV) in their sera. To generate a suitable antigen, peptidyl arginine deiminase (PAD) enzymes are needed to deiminate vimentin, which is usually not in a citrullinated state.[9] Vimentin in macrophages is diminished, consequently, PAD-induced apoptosis, which brings calcium into the cell.[10] Therefore, when macrophages and other cells undergo apoptosis, citrullinated vimentin is produced in the synovial fluid. Vimentin functions through signaling cascades, including the P13k pathway and extracellular kinase, which trigger Glycogen synthase kinase-3beta (GSK3β). This, in turn, causes an increase in proinflammatory cytokines, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) interleukins (ILs), including IL-6, and nuclear factor-kB ligand-receptor activator. When released into the extracellular space during disease, citrullinated vimentin stimulates different innate immune cells. Vimentin that has had its glycine to arginine substitution changed is known as mutant vimentin, and it is believed that this change is brought on by oxidative stress. Vimentin mutations can result in cytoplasmic aggregate-like formations of different sizes. An elevated level of MCV antibodies in the blood is significantly associated with RA.
An antibody to MCV is a sensitive and specific biomarker for RA diagnosis. The presence of anti-MCV seems to be a predictor of RA. Anti-MCV antibody testing is primarily beneficial because it detects anti-MCV antibodies early, allowing for early diagnosis of RA and prompt initiation of appropriate therapy.[11] High sensitivity and specificity have been demonstrated in the measurement of anti-MCV IgG isotype autoantibodies using the enzyme-linked immunosorbent assay (ELISA) for RA diagnosis.[12] The study by Nicaise Roland et al. indicates that anti-MCV can be used to diagnose anti-CCP-negative RA patients and monitor their response to infliximab therapy.[11] This study is designed to investigate the diagnostic utility of serum anti-MCV antibodies in seropositive and seronegative RA patients. The second objective was to ascertain the specificity and sensitivity of MCV antibodies for RA diagnosis and other connective tissue disorders (CTDs), as well as the effectiveness of serum MCV antibodies in picking out RA over anti-CCP or RF.
MATERIALS AND METHODS
Participants and study design
It is a cross-sectional study. The study was carried out at Nizam’s Institute of Medical Sciences, Punjagutta, Hyderabad, in collaboration with the Department of Clinical Immunology and Rheumatology and the Department of Biochemistry from March 2020 to March 2021.
Inclusion criteria
The study included 60 RA patients aged 18–70 years and 60 age-appropriate controls. In the control group, there were 52 females and 8 males, while in the patient group, there were 51 females and 9 males. Following RF and anti-CCP testing, cases were distributed into two groups. Thirty patients from Group I were seropositive and had results that were either RF or anti-CCP positive, while thirty patients from Group II were seronegative and had results that were both anti-CCP and RF negative. Thirty healthy volunteers and thirty patients with other CTDs with RF positive constitute the sixty controls.
Exclusion criteria
Patients with diabetes mellitus, chronic hypertension, heart failure, myocardial infarction, asthma, chronic kidney disease, other infections, and known cases of cancer are excluded from the study.
Laboratory tests
Serum aliquots were stored at −20°C. Anti-MCV and anti-CCP tests were carried out in a batch of 20 samples using enzyme-linked immunosorbent assay kits procured from Krishgen Biosystems, USA. The anti-MCV assay’s basic method involves quantifying human anti-mutated vimentin antibody levels in human serum in vitro using ELISA. The anti-MCV assay has an analytical range of 0.4–12.8 ng/mL. The specificity and sensitivity of the anti-MCV and anti-CCP tests were evaluated using the criteria of the American College of Rheumatology. The complete blood count, erythrocyte sedimentation rate (ESR), and RF results were taken out of the patient records.
Statistical analysis
Version 9 of GraphPad Prism was utilized for doing the statistical analysis. We employed the Kolmogorov–Smirnov test to confirm the normality of each control and case variable. If the data is not parametric, it is expressed as median (interquartile range); otherwise, it is reported as mean ± Standard deviation (SD). When expressing nominal data, the percentage is applied. The one-way analysis of variance or Student’s t-test was used to compare normally distributed data between groups; the Kruskal–Wallis or Mann–Whitney U-tests were used to analyze non-normally distributed data. To investigate the categorical variables between the groups, the Fisher’s exact or Chi-square test was employed. To study associations between variables, Spearman’s correlation test is used. Following the construction of receiver operating characteristic (ROC) curves, the following parameters were determined: Sensitivity, specificity, and area under the curve (AUC). A value of P < 0.05 was utilized to show statistical significance.
RESULTS
Nine cases (15%) were male, and 51 cases (85%) were female. In the control group, there were sixty people; 51 (85%) were female and 9 (15%) were male. Table 1 shows the test results and demographic information for both patients and controls. There was no statistically significant change in the age distribution (P = 0.36). Furthermore, no significant difference (P = 0.13, P = 0.21, respectively) was observed between the patient and control groups for creatinine, alanine aminotransferase, or gender. Hemoglobin, aspartate aminotransferase, and total leukocyte count, on the other hand, showed a significant difference between the control and patient groups (P < 0.001, P = 0.02, and P < 0.001, respectively).
| Controls | Seropositive CTD | Seropositive RA (n=30) | Seronegative RA | P-value | |
|---|---|---|---|---|---|
| (n=30) | (n=30) | (n=30) | (n=30) | ||
| Age (years) | 31 (29–35) | 32 (26–42) | 34 (27–45) | 35 (30–41) | 0.3658 |
| Male (%) | 5 (17) | 4 (13) | 6 (20) | 2 (6.6) | |
| Female (%) | 25 (83) | 26 (87) | 24 (80) | 28 (93.3) | |
| Duration (months) | _ | 12 (6–24) | 10 (6–12) | 1 (0.5–2.15) | 0.1703 |
| AST (U/L) | 18 (15–22.25) | 25 (20–35) | 20.0 (17–24) | 16 (13.75–21.25) | <0.001 |
| ALT (U/L) | 15 (11–18.5) | 20.0 (13–34) | 17.5 (11.75–33) | 14 (10.75–23) | 0.1359 |
| Creatinine (mg/dL) | 0.6 (0.52–0.67) | 0.54 (0.4–0.7) | 0.54 (0.41–0.66) | 0.61 (0.48–0.69) | 0.2193 |
| Hemoglobin (g/dL) | 11.2 (10.20–12.13) | 11.10 (10.18–11.93) | 11.3 (10.45–12.03) | 12.10 (11.18–13.23) | 0.0227 |
| ESR (mm/1st h) | 6.60 (2.0–9.25) | 16.5 (10–47) | 19.0 (9.0–38) | 23 (15–35.25) | <0.0001 |
| TLC (cell/mL) | 7,300 (6,200–8,350) | 3,300 (2,400–5,650) | 5,625 (4,350–8,575) | 8,900 (7.300–10.600) | <0.0001 |
| RF (IU/mL) | 7 (2.70–12.0) | 110.5 (60–198) | 214 (109.8–301.5) | 3 (1–12.25) | <0.0001 |
| Anti-CCP (pg/mL) | 34.69 (31.26–38.19) | 96.55 (91.67–101.7) | 534.5 (470.1–568.3) | 93.13 (88.6–99.42) | <0.0001 |
| Anti-MCV (ng/mL) | 2.150 (1.297–2.403) | 5.39 (3.249–7.11) | 7.985 (7.221–14.10) | 10.51 (9.756–10.86) | <0.0001* |
Significant differences were seen in the median RF titers across the four groups: Healthy controls, CTD controls, seronegative RA cases, and seropositive RA cases. RF titers are significantly higher in seropositive cases (P < 0.0001) than in seronegative cases and controls. There is no significant difference in RF titers between seronegative patients and controls (P > 0.999). The median anti-CCP titers varied significantly among the four groups (P = 0.0069). Seropositive people had significantly greater levels of anti-CCP than controls (P = 0.0062) and seronegative cases (P < 0.0001). However, there is no significant difference in the anti-CCP concentration between controls and seronegative patients (P > 0.999).
The median values of anti-MCV, anti-CCP, and RF are shown in Figure 1. The median serum concentrations of anti-MCV varied between the four groups. Compared to controls, there is a significant increase in anti-MCV in both seronegative and seropositive cases (P < 0.0001). There was no significant change in anti-MCV levels between seronegative and seropositive cases, despite the greater levels in seronegative cases (P > 0.99). Fisher’s exact test shows that the distribution of anti-MCV in seronegative and seropositive cases does not differ statistically, suggesting that the biomarker is increased similarly in both cases. The results of the post hoc analysis of anti-MCV, RF, and anti-CCP are given in Table 2.
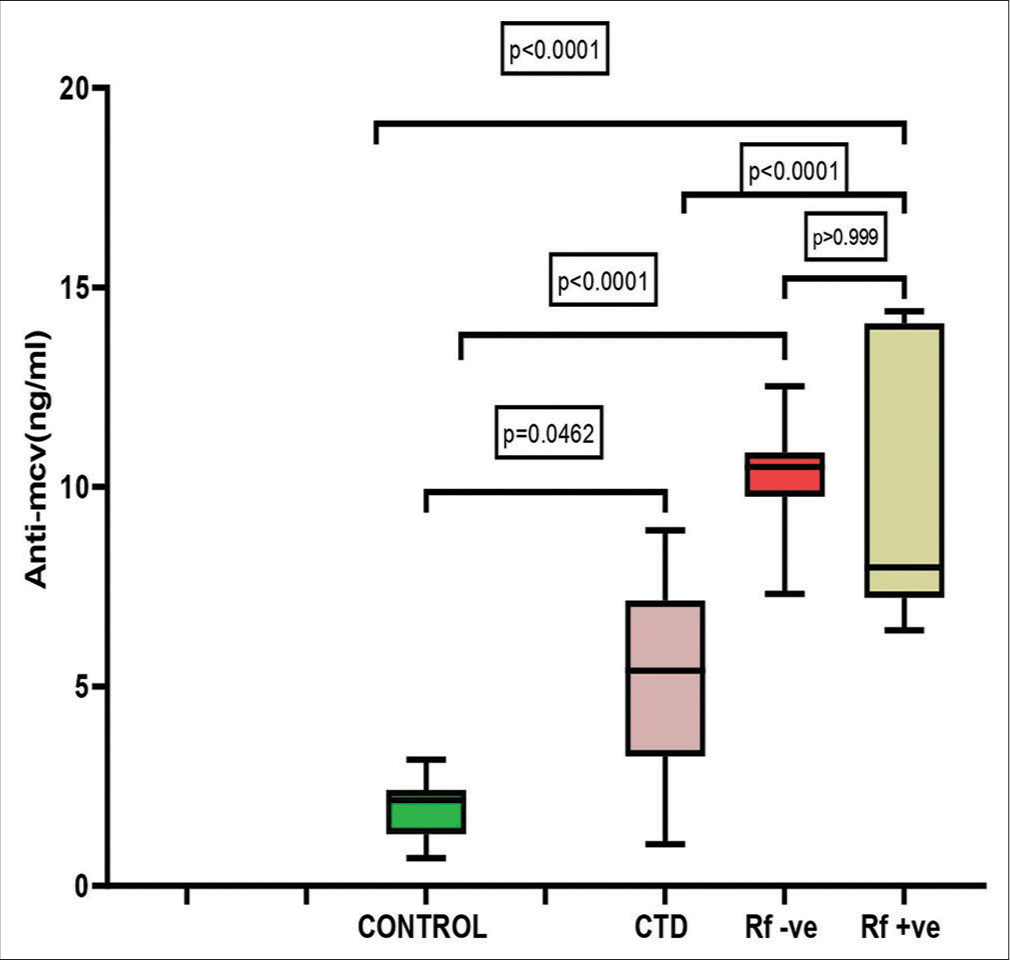
- Comparison of anti-mutated citrullinated vimentin levels between controls, connective tissue disorder cases, seronegative rheumatoid arthritis (RA) cases, and seropositive RA cases. MCV: Mutated citrullinated vimentin, CTD: Connective tissue disorder, Rf: Rheumatoid factor.
| Variable | Control versus CTD | CTD versus seronegative RA | Seronegative RA versus seropositive RA | Seropositive RA versus CTD | Seropositive RA versus controls | Seronegative RA versus control | Seronegative RA versus CTD |
|---|---|---|---|---|---|---|---|
| Anti- MCV | 0.0462 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | >0.9999 | <0.0001 |
| RF | <0.0001 | <0.0001 | <0.0001 | 0.9110 | <0.0001 | >0.9999 | <0.0001 |
| Anti-CCP | <0.0001 | >0.999 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | >0.9999 |
RF: Rheumatoid factor, Anti-CCP: Anti-cyclic citrullinated protein antibody, Anti-MCV: Anti-mutated citrullinated vimentin, CTD: Connective tissue disorder, RA: Rheumatoid arthritis
The diagnostic accuracy of anti-MCV in patients with seronegative and seropositive RA was assessed by ROC curve analysis. At an ideal cutoff of 6.35 ng/mL, anti-MCV showed 100% sensitivity and 83.33% specificity in cases with seropositive RA; in cases of seronegative RA, these values were 83.33% and 100%, respectively. Figure 2 shows that the anti-MCV combined ROC curve analysis showed 100% sensitivity, 83.33% specificity, and 0.97 AUC in cases of seronegative and seropositive RA. The RF and anti-CCP ROC analyses revealed AUCs of 0.84 and 0.54, respectively [Figure 3]. Table 3 demonstrates the diagnostic performance (sensitivity, specificity, and AUC) of the anti-MCV, RA, and anti-CCP assays.
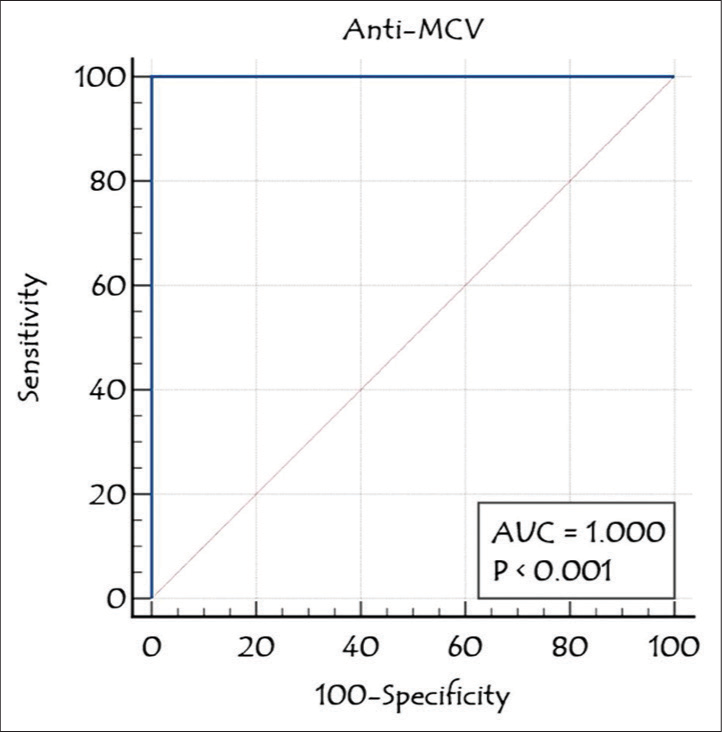
- Receiver operating characteristic analysis of anti-mutated citrullinated vimentin in rheumatoid arthritis cases. Anti-MCV: Anti-mutated citrullinated vimentin, AUC: Area under the curve.
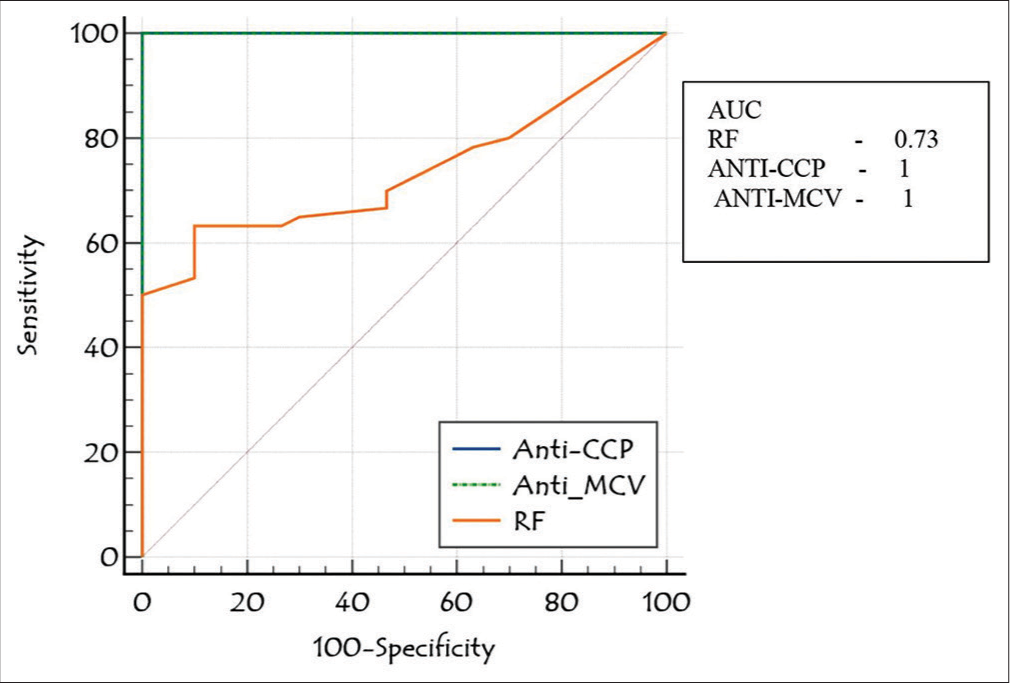
- Receiver operating curve comparison of anti-mutated citrullinated vimentin with rheumatoid factor and anti-cyclic citrullinated protein antibody in rheumatoid arthritis patients. RF: Rheumatoid factor, Anti-CCP: Anti-cyclic citrullinated protein antibody, Anti-MCV: Anti-mutated citrullinated vimentin, AUC: Area under the curve
| Cut-off | AUC | Sensitivity | Specificity | |
|---|---|---|---|---|
| RF (n=90) | >20 (IU/mL) | 0.73 | 50 | 50 |
| anti-CCP (n=90) | >103.84 (pg/mL) | 1.0 | 100 | 91 |
| anti-MCV (n=90) | >3.163 (ng/mL) | 1.0 | 100 | 100 |
RF: Rheumatoid factor, Anti-CCP: Anti-cyclic citrullinated protein antibody, Anti-MCV: Anti-mutated citrullinated vimentin, AUC: Area under the curve, RA: Rheumatoid arthritis
Based on the ROC cutoff, subjects were categorized as either anti-MCV negative or anti-MCV positive. Anti-MCV is positive in all 60 RA cases. Out of the 60 controls, eleven tested positive for anti-MCV, whereas the remaining 49 tested negative. Table 4 shows the subjects’ distribution according to the anti-MCV. The novel marker anti-MCV demonstrated 100% specificity and sensitivity with 100% negative predictive value (NPV) and positive predictive value (PPV) in RA patients.
| Control (n=30) | CTD cases (n=30) | Seropositive RA cases (n=30) | Seronegative RA cases (n=30) | |
|---|---|---|---|---|
| Anti-MCV positive (>3.16 ng/mL)* (%) | 30 (100) | 23 (76.6) | 30 (100) | 30 (100) |
| Anti-MCV negative (<3.16 ng/mL) | 0 | 7 (23.3%) | 0 | 0 |
It showed 36.67% sensitivity and 100% specificity among RF-positive CTD controls, with a 61.2% PPV and 100% NPV [Table 5]. In patients of seronegative and seropositive RA, there is no significant correlation between serum anti-MCV (ρ = 0.1812, P = 0.33). When anti-MCV was correlated with RF in RA cases, there was no significant correlation (ρ = 0.1605, P = 0.79) [Figure 4]. Figure 5 illustrates a statistically significant correlation (ρ = 0.5860, P < 0.0001) between anti-CCP and RA patients. When correlation analysis of anti-MCV was done with other variables, anti-CCP, total count, and ESR were revealed to be significant independent RA predictors (P = 0.0001, 0.028, and 0.0001, respectively). Logistic regression of anti-MCV gave a P = 0.0060, which is a predictor of RA.
| Biomarker | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Anti-MCV in RA cases | 100 | 100 | 100 | 100 |
| Anti-MCV in RF-positive CTD cases (>3.16 ng/mL) | 76.6 | 100 | 81 | 100 |
RA: Rheumatoid arthritis, RF: Rheumatoid factor, Anti-MCV: Anti-mutated citrullinated vimentin, CTD: Connective tissue disorder, PPV: Positive predictive value, NPV: Negative predictive value
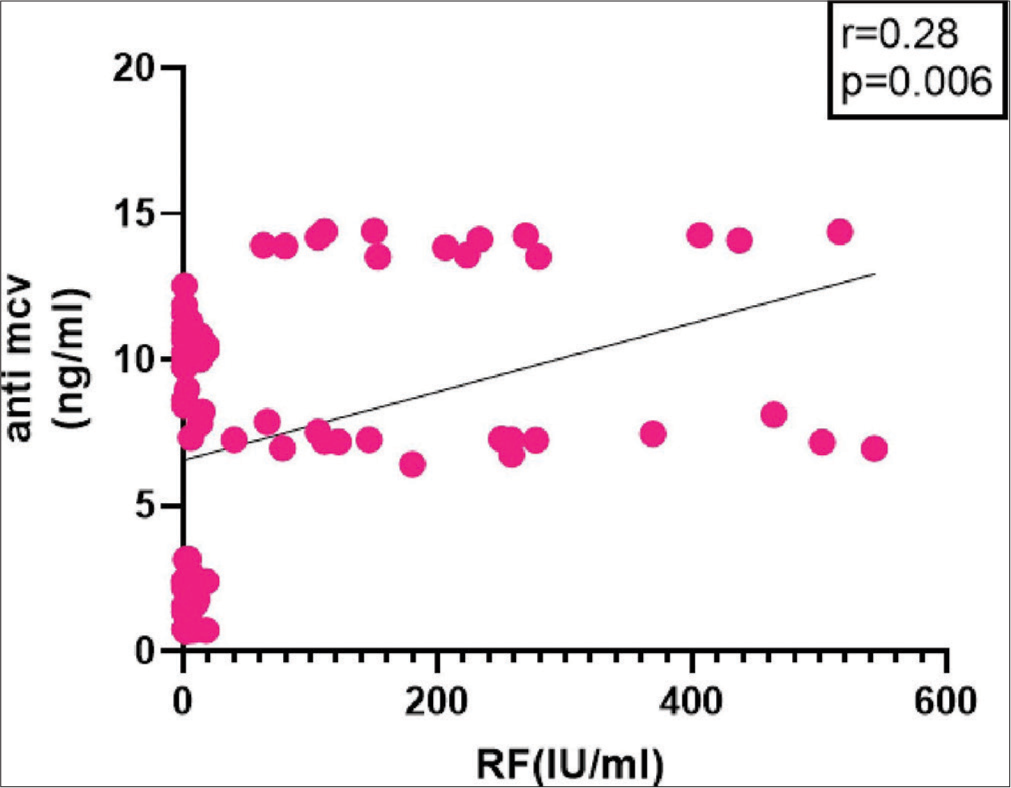
- Correlation of anti-mutated citrullinated vimentin with rheumatoid factor in rheumatoid arthritis cases and controls. RF: Rheumatoid factor, Anti-MCV: Anti-mutated citrullinated vimentin
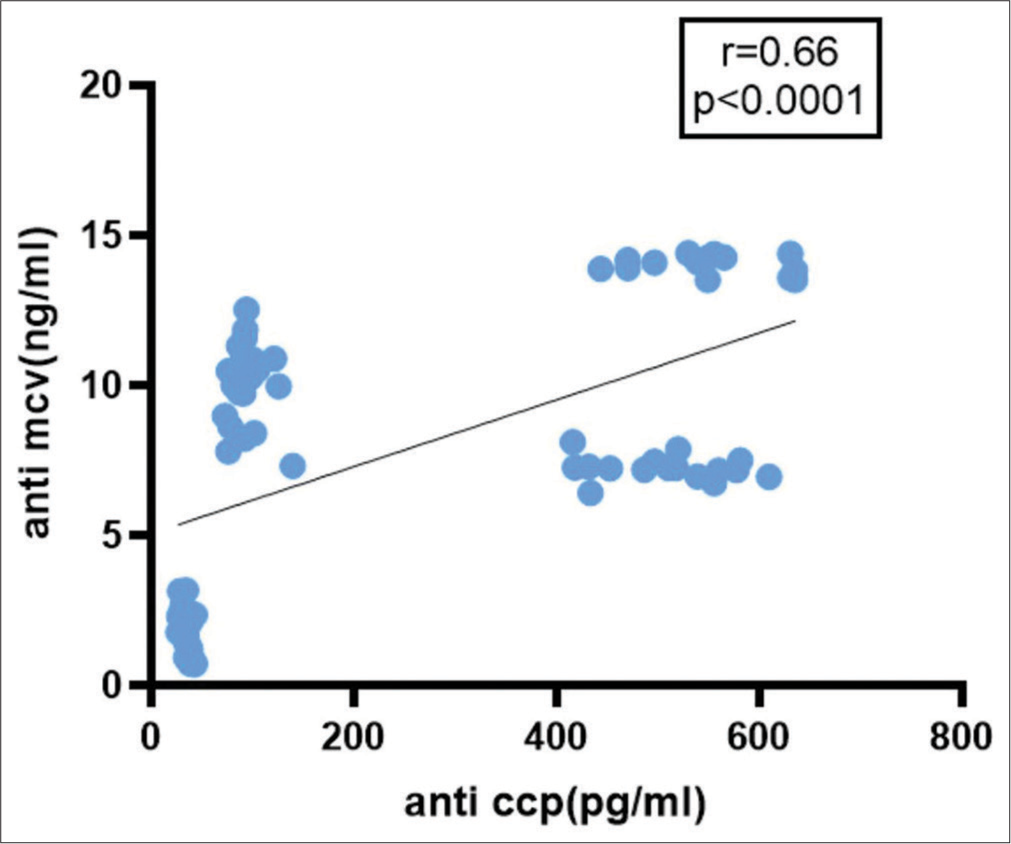
- Correlation of anti-mutated citrullinated vimentin with anti-cyclic citrullinated protein antibody in rheumatoid arthritis cases and controls. Anti-CCP: Anti-cyclic citrullinated protein antibody, Anti-MCV: Anti-mutated citrullinated vimentin
DISCUSSION
A persistent autoimmune-inflammatory joint disease is RA. Although advances in treatment have been achieved, the diagnosis of RA remains a challenge to physicians. The existing biomarkers do not satisfactorily identify the RA. This study’s main goal was to determine the specificity and sensitivity of anti-MCV in Indian patients with seronegative and seropositive RA when combined with RF and anti-CCP. We observed that anti-MCV possesses the maximum sensitivity when compared to RF and anti-CCP. A combination of clinical symptoms, clinical findings, and laboratory studies is used to diagnose RA. The usefulness of RF, anti-CCP, and other promising biomarkers for RA early identification has been assessed in numerous investigations. While anti-CCP is a specific marker, RF is a sensitive marker that can identify a greater proportion of patients in the current examination panel. [13,14] Anti-CCP has a sensitivity of only 40% in individuals with early RA, and its expression in synovial fluid is undetectable.[15]
To detect more cases of RA and prevent the disease’s development, researchers are looking into additional biomarkers due to the current diagnostic panel’s lack of sensitivity and specificity. Growth factors and other proinflammatory cytokines cause increased production of citrullinated vimentin, which could be involved in RA pathogenesis. During synovial and joint destruction, anti-MCV is produced far earlier than anti-CCP. Autoantibodies against citrullinated vimentin interact with the osteoclast lineage to cause joint damage by promoting loss of periarticular bone.[16] According to Sghiri et al., anti-MCV antibodies exhibit superior sensitivity while maintaining the same specificity as anti-CCP antibodies.[17] Dejaco et al. observed that anti-MCV has similar performance characteristics to anti-CCP for diagnosing RA.[18]
A few studies using anti-MCV for RA diagnosis reported similar performance characteristics to anti-CCP. Assessing serum anti-MCV prevalence and diagnostic usefulness in RA was our primary objective. Thus, in RA cases, the anti-MCV serum levels were evaluated. Its great specificity for RA is the most advantageous feature of anti-CCP. RF positives with primary diagnoses other than RA were compared to ascertain the anti-MCV specificity in RA. Dimer vimentin is made up of flexible head and tail domains surrounding an α- helical “rod” domain of coiled coils. The three α-helical segments (coils 1A, 1B, and 2) that comprise this structure are joined by linkers L1 and L12. The rod domain of all cytoplasmic intermediate filament (IF) proteins is around 310 residues long, but the rod domain of nuclear IF proteins is about 350 residues long.[19] Vimentin is a normal intermediary filament seen in the synovial fluid. The release of vimentin in serum is little, but large quantities of citrullinated vimentin are released only after tissue injury, inflammation, and apoptosis. Vimentin is easily detectable in the synovium and is abundantly expressed by macrophages and mesenchymal cells. When macrophages undergo apoptosis, the protein is altered. If the apoptotic material is not eliminated, citrullinated vimentin antibodies may develop.
Vimentin functions through signaling cascades that include the P13k pathway and extracellular kinase. This initiates the activation of GSK3β, which subsequently upregulates proinflammatory cytokines such as IL-6, NF-kB, ILs, and receptor activator of nuclear factor kappa beta (RANK) ligands. When disease conditions release citrullinated vimentin into the extracellular space, it stimulates different innate immune cells. Antibodies against citrullinated vimentin are useful indicators for monitoring RA progression. The main advantage of anti-MCV testing is the early production of anti-MCV antibodies in patients with very early RA. In addition, MCV antibody titers exhibit a strong correlation with disease activity, disease severity, and therapeutic efficacy.[20]
To our knowledge, no research conducted in India has looked at the role of circulating anti-MCV in patients with seronegative or seropositive RA. The median anti-MCV levels in RA cases are substantially higher than in control groups. In a study by Abou-Elfattah Tawfik et al., the patients’ mean ± SD anti-MCV levels (61.8 ± 47.1 ng/mL) were markedly higher than the control group (8.3 ± 2.9 ng/mL), suggesting a case of RA. The sensitivity, specificity, PPV, and NPV of anti-MCV are all 92%.[21] The lower levels of anti-MCV in our study may have resulted from the exclusion of patients and controls with co-morbid inflammatory disease conditions when anti-MCV levels are increased in the blood. One possible explanation for their study’s lesser sensitivity could be higher cut-offs. The median RF levels in RA cases are significantly higher than in the control group. The median anti-CCP levels were significantly different between the two groups. Anti-MCV, RF, and anti-CCP ROC analyses are performed; the resulting AUC values are 0.97, 0.54, and 0.84, respectively.
In our study, we also observed that anti-MCV is equally raised in both seropositive and seronegative cases with high sensitivity and specificity. In contrast, Shah et al. demonstrated that anti-MCV had a low sensitivity (32.9%) when used to diagnose seronegative RA.[22] According to Maraina et al., anti-MCV antibody is not a more useful biomarker for diagnosing RA patients than RF or anti-CCP. RF, anti-CCP, and anti-MCV had sensitivity and specificity values of 85%, 71%, 80%, 75%, 95%, and 60%, respectively.[23] In comparison to anti-MCV antibodies (87%), RF (94%), and other antibodies, anti-CCP antibodies demonstrated the best specificity (97%) and PPV (0.93) in an Omani study. Conversely, anti-MCV antibodies showed the highest sensitivity (72%), in contrast to RF (57%) and anti-CCP antibodies (52%). When anti-MCV antibody sensitivity was compared to anti-RF and anti-CCP antibody sensitivity, a significant difference was found (P = 0.023).[24]
Based on the assay’s 84% sensitivity and 80% specificity, the authors of an Egyptian study concluded that there is a significant correlation between the radiologic progression of RA and the anti-MCV assay.[25] The authors of another Egyptian study reported that the PPV, NPV, sensitivity, and specificity of anti-MCV in RA are 97.8, 99.8, 99.1, and 93.3, respectively. Anti-MCV had an AUC value of 0.997. They found that, particularly in situations where other markers are negative, anti-MCV is a useful diagnostic with high sensitivity and specificity for the early detection of RA.[26] Lipinska et al. have demonstrated the diagnostic and prognostic importance of anti-MCV and anti-CCP in juvenile idiopathic arthritis (JIA) in children. Of the 30 JIA children, 11 (36.6%) tested positive for anti-MCV, while 12 (40%) tested positive for anti-CCP.[27]
In meta-analysis research, the combined summary values of anti-CCP antibody and RF positivity were 96% specificity, 57% sensitivity, 0.46 negative likelihood ratio (LR), 33.84 positive likelihood ratio, and 33.02 diagnostic odds ratio for the diagnosis of RA.[28] Thirty of the seronegative RA patients in our study tested positive for anti-MCV. Therefore, our study’s findings on the prevalence of anti-MCV in the seronegative group have not been found in any other studies. Furthermore, we discovered that anti-MCV had a significant association with anti-CCP in RA cases but no positive correlation with RF. In addition, we also noticed that anti-MCV and other RA indicators, such as total leukocyte count (TLC) and ESR, had a positive correlation. A study by Osman et al. demonstrated a strong positive correlation between anti-CCP and anti-MCV in RA patients.[26] Few other studies found a similar positive correlation between anti-CCP and anti-MCV in RA cases.[25,29-31]
Limitations
The study’s two main limitations are the small sample size and the lack of a follow-up analysis.
CONCLUSIONS
In summary, RA cases had higher median anti-MCV levels than CTD controls and healthy controls. In addition, compared to seropositive RA cases, anti-MCV levels were greater in seronegative RA cases. Anti-MCV, a sensitive marker, can be used to diagnose RA in seropositive or seronegative patients. Compared to RA patients, the median anti-MCV levels in CTD controls are lower. Anti-MCV can, therefore, greatly enhance the diagnosis of RA in CTD cases. In patients, especially those who are seronegative for either biomarker, anti-MCV, when combined with RF and anti-CCP biomarkers, may help diagnose more RA cases. More extensive patient-based studies are required to prove that this novel biomarker is a sensitive, specific, and predictive serological biomarker for the early diagnosis of RA.
Acknowledgments
We thank our laboratory technician, V. Naga Rani, for her technical assistance and valuable input.
Author contribution
DS: Literature search, experimental studies, data acquisition; K.S.S.SB: Concept, statistical analysis; SD: The definition of intellectual content, literature search; LR: Design, manuscript review; MVB: Experimental studies, manuscript preparation; NM: Data acquisition, data analysis; SAK: Literature search, manuscript editing; N.NS: Statistical analysis, manuscript preparation; IKM: Concept, design, data analysis, manuscript editing, manuscript review.
Ethical approval
The study was approved by the Nizam’s Institute of Medical Sciences Ethical Committee (EC/NIMS/2477/2019).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Antibodies against a mutated citrullinated vimentin in patients with rheumatoid arthritis. Egypt J Immunol. 2022;29:184-94.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of rheumatoid arthritis in the adult Indian population. Rheumatol Int. 1993;13:131-4.
- [CrossRef] [PubMed] [Google Scholar]
- Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15.
- [CrossRef] [PubMed] [Google Scholar]
- Update on the diagnosis and management of early rheumatoid arthritis. Clin Med (Lond). 2020;20:561-4.
- [CrossRef] [PubMed] [Google Scholar]
- Anticitrullinated protein/peptide antibody and its role in the diagnosis and prognosis of early rheumatoid arthritis. Neth J Med. 2002;60:383-8.
- [Google Scholar]
- Anti-microRNA-378a enhances wound healing process by upregulating integrin beta-3 and vimentin. Mol Ther. 2014;22:1839-50.
- [CrossRef] [PubMed] [Google Scholar]
- Vimentin knockdown decreases corneal opacity. Invest Ophthalmol Vis Sci. 2014;55:4030-40.
- [CrossRef] [PubMed] [Google Scholar]
- Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis. 2004;63:373-81.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-Sa antibodies: Prognostic and pathogenetic significance to rheumatoid arthritis. Arthritis Res Ther. 2004;6:86-9.
- [CrossRef] [PubMed] [Google Scholar]
- Antibodies to mutated citrullinated vimentin for diagnosing rheumatoid arthritis in anti-CCP-negative patients and for monitoring infliximab therapy. Arthritis Res Ther. 2008;10:R142.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-cyclic citrullinated peptide (anti-CCP) and anti-mutated citrullinated vimentin (anti-MCV) relation with extra-articular manifestations in rheumatoid arthritis. J Immunol Res. 2014;2014:536050.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction models for rheumatoid arthritis during diagnostic investigation: Evaluation of combinations of rheumatoid factor, anti-citrullinated protein/peptide antibodies and the human leucocyte antigen-shared epitope. Ann Rheum Dis. 2007;66:364-9.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value of anti-human citrullinated fibrinogen ELISA and comparison with four other anti-citrullinated protein assays. Arthritis Res Ther. 2006;8:R122.
- [CrossRef] [PubMed] [Google Scholar]
- Antibodies against citrullinated peptide (CCP) and levels of cartilage oligomeric matrix protein (COMP) in very early arthritis: Relation to diagnosis and disease activity. Scand J Rheumatol. 2004;33:185-8.
- [CrossRef] [PubMed] [Google Scholar]
- Periarticular bone loss in arthritis is induced by autoantibodies against citrullinated vimentin. J Bone Miner Res. 2017;32:1681-91.
- [CrossRef] [PubMed] [Google Scholar]
- Value of anti-mutated citrullinated vimentin antibodies in diagnosing rheumatoid arthritis. Rheumatol Int. 2008;29:59-62.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value of antibodies against a modified citrullinated vimentin in rheumatoid arthritis. Arthritis Res Ther. 2006;8:R119.
- [CrossRef] [PubMed] [Google Scholar]
- Atomic structure of the vimentin central α-helical domain and its implications for intermediate filament assembly. Proc Natl Acad Sci U S A. 2012;109:13620-5.
- [CrossRef] [PubMed] [Google Scholar]
- Antibodies against citrullinated vimentin in rheumatoid arthritis: Higher sensitivity and extended prognostic value concerning future radiographic progression as compared with antibodies against cyclic citrullinated peptides. Arthritis Rheum. 2008;58:36-45.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-mutated citrullinated vimentin antibodies (Anti-MCV): A relation with other diagnostic markers in rheumatoid arthritis patients. Clin Med Diagn. 2019;9:61-7.
- [Google Scholar]
- Diagnostic usefulness of anti-cyclic citrullinated peptide and anti-mutated citrullinated vimentin antibodies in the diagnosis of seronegative rheumatoid arthritis patients. J Islam Int Med Coll. 2016;11:3-7.
- [Google Scholar]
- Diagnostic value of anti-modified citrullinated vimentin in rheumatoid arthritis. Int J Rheum Dis. 2010;13:335-9.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of anti-mutated citrullinated vimentin antibodies, anti-cyclic citrullinated Peptide antibodies and rheumatoid factor in omani patients with rheumatoid arthritis. Int J Rheumatol. 2012;2012:285854.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-mutated citrullinated vimentin antibodies in rheumatoid arthritis patients: Relation to disease activity and manifestations. Egypt Rheumatol. 2014;36:65-70.
- [CrossRef] [Google Scholar]
- Anti-mutated citrullinated vimentin (Anti-MCV) antibodies as a diagnostic aid for rheumatoid arthritis. J Clin Cell Immunol. 2014;5:5.
- [CrossRef] [Google Scholar]
- Anti-MCV and anti-CCP antibodies-diagnostic and prognostic value in children with juvenile idiopathic arthritis (JIA) Clin Rheumatol. 2016;35:2699-706.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of combined tests of anti cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis: A meta-analysis. Clin Exp Rheumatol. 2014;32:11-21.
- [Google Scholar]
- The anti-mutated citrullinated vimentin response classifies patients with rheumatoid arthritis into broad and narrow responders. J Rheumatol. 2009;36:2670-4.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative performance analysis of 4 different anti-citrullinated protein assays in the diagnosis of rheumatoid arthritis. J Rheumatol. 2009;36:491-500.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-mutated citrullinated vimentin antibodies in rheumatoid arthritis compared with anti-cyclic citrullinated peptides. J Am Sci. 2013;9:160-6.
- [Google Scholar]






