Translate this page into:
Effect of Different Anticoagulants on HbA1C Estimation and its Stability
Address for correspondence: Dr. Booloo Sharma, E-mail: sharmabooloo@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Glycated hemoglobin/HbA1c is always considered as a stable indicator of glycaemia for the preceding three months.[1] Its potential utility in diabetic care was first reported in 1985 WHO report, and by 2010 all the major expert committee and association across the globe including the ADA has recommended HbA1c for the diagnosis of Type 2 DM, besides its role in prognosis.[2] So, the importance of HbA1c estimation in diabetes has increased manifold in recent years.
Most of the commercial kits for HbA1c estimation requires sample to be collected in EDTA anticoagulant. This requires additional sample collection for the patient. So, we estimated HbA1c in sample collected in K3 EDTA, Na-citrate, Na-heparin and Na-fluoride/Na2 EDTA vials separately and then to see for any variation. All the vials used are of BD (Becton, Dickinson and Company) Vacutainer manufactured by Becton, Dickinson India. HbA1c is estimated after 2-3 hours of blood collection by cation exchange HPLC based BioRad D 10 analyzer. It is said that HbA1c has high pre-analytical stability and is stable for one week when stored at 4°C and for one year when stored at −70°C.[34] So, to verify the stability of HbA1c in the above vials HbA1c is estimated for 7 days in the same vial. Just after estimation, vials are stored at 4°C and then HbA1c measured every day till 7th day in all the vials, marking 1st day as 0 day.
As indicated by Figures 1–6, there is no significant change in the HbA1c values estimated in the vials containing different anticoagulant. This is in accordance with the findings of Mailankot et al.[5] Figures 1–6, also shows that the stability of HbA1c is not altered in the vials containing different anticoagulant when stored at 4°C for 7 days. The findings withdraws the need for a separate EDTA sample for HbA1c estimation as advocated by various commercial kits, and it also ensures the stability of the collected sample for 7 days when stored at 4°C.

- The results for healthy non-diabetic adult samples
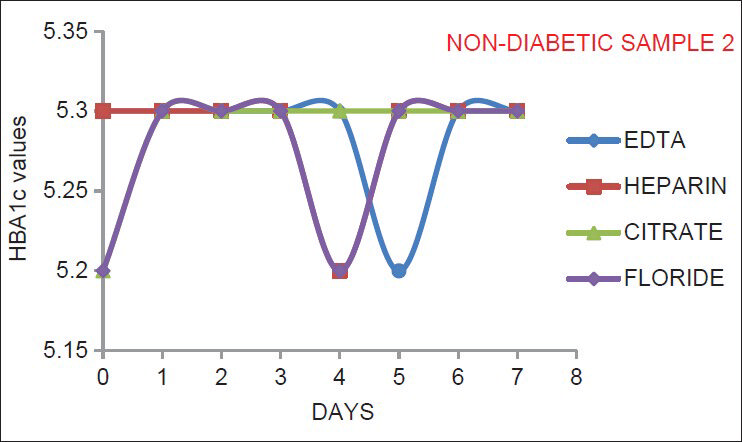
- The results for healthy non-diabetic adult samples
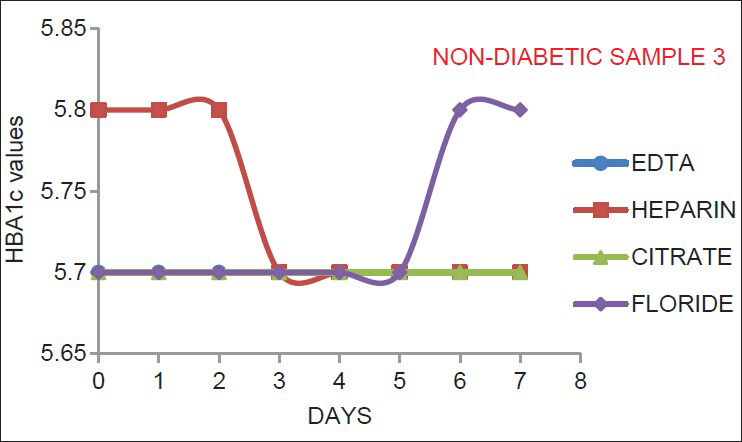
- The results for healthy non-diabetic adult samples
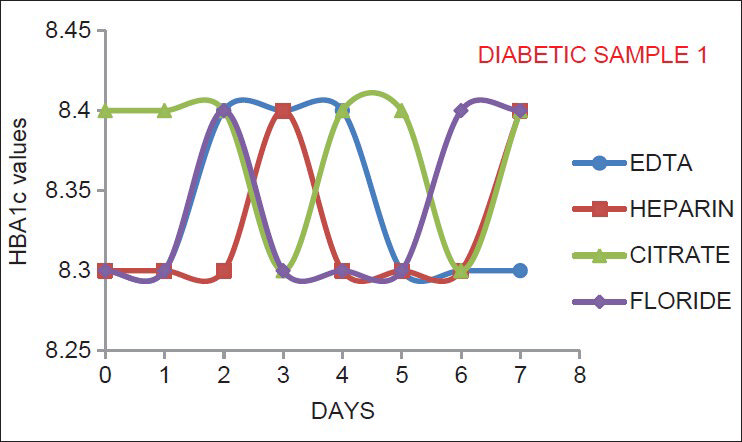
- The results for adult diabetic samples
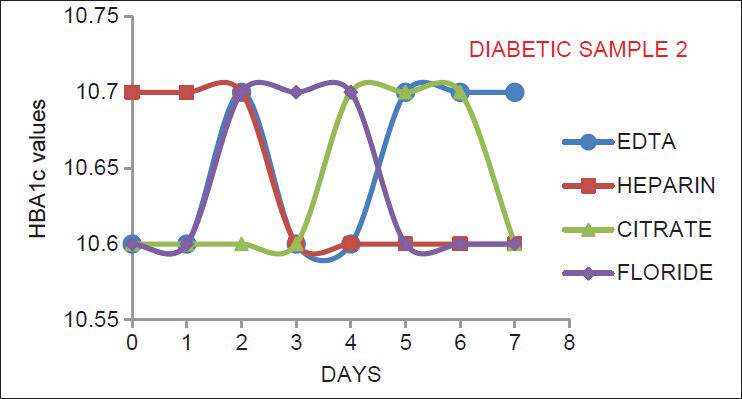
- The results for adult diabetic samples
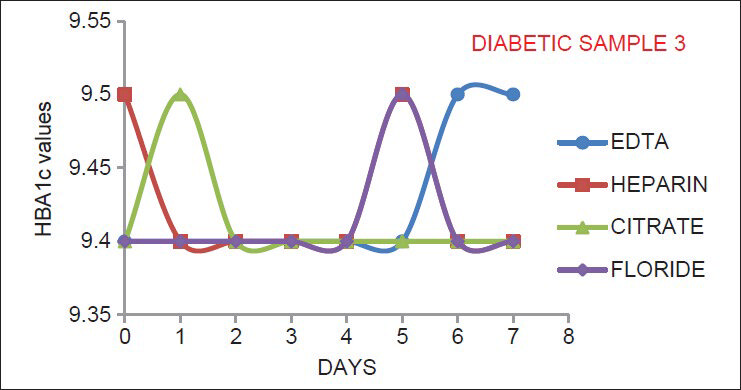
- The results for adult diabetic samples
To conclude, we can infer that the unnecessary extra sample collection for estimation of HbA1c could be avoided which in turn can improve patient compliance and this may contribute towards a better diabetic care. It can also be accounted that the avoidance of an extra Vacutainer for HbA1c can reduce the cost of HbA1c test, which is undoubtedly a costly test.
REFERENCES
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64-71.
- [Google Scholar]
- Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48:436-72.
- [Google Scholar]
- Effects of sample storage conditions on glycated hemoglobin measurement: Evaluation of five different high performance liquid chromatography methods. Diabetes Technol Ther. 2007;9:36-42.
- [Google Scholar]
- Various anticoagulants and fluoride do not affect HbA1C level. Ind J Clin Biochem. 2012;27:209.
- [Google Scholar]




