Translate this page into:
Immunohistochemical characterization of inflammatory infiltrates in unstable vitiligo
*Corresponding author: Amit Kumar Yadav, Department of Pathology, Vardhman Mahavir Medical College and Safdarjung Hospital, Ansari Nagar, New Delhi, India. path.yadav@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singh P, Mishra P, Yadav AK, Khunger N. Immunohistochemical characterization of inflammatory infiltrates in unstable vitiligo. J Lab Physicians. 2024;16:358-65. doi: 10.25259/JLP_29_2023
Abstract
Objectives:
Disease instability in vitiligo is a prominent step during the development or extension of disease. The presence of marked inflammatory infiltrate may be considered a diagnostic clue for disease instability. However, there is a paucity of literature regarding this. Therefore, the present study was carried out to characterize the nature of inflammatory infiltration in cases of unstable vitiligo.
Materials and Methods:
Thirty patients of unstable vitiligo diagnosed clinically were enrolled and two biopsies: Lesional and perilesional obtained. Histopathological examination with respect to five parameters, i.e., spongiosis, epidermal lymphocytes, basal cell vacuolation, dermal lymphocytes, and melanophages was done including histological scoring. Immunohistochemical characterization was done for T lymphocytes, Langerhans cells (LCs), macrophages, and B cells by studying their number and distribution.
Statistical analysis:
Statistical analysis was done using Spearman’s rank coefficient correlation test.
Results:
Mean T-lymphocytes, macrophages, and LC count were significantly higher in lesional skin. The three parameters correlated with vitiligo histological score. T cells were present more frequently in the dermis and stratum basale. Macrophages were found more in the dermis whereas LC was mainly located in the epidermis.
Conclusions:
An increase in the population of inflammatory cells, especially T lymphocytes and LC, may serve as an indicator of unstable vitiligo. The relative distribution of these cells points toward signaling between them and their role in the destruction of melanocytes and keratinocytes. A better understanding of the underlying mechanisms may lead to the development of novel targeted therapies.
Keywords
B cells
Langerhans cells
T cells
Unstable vitiligo
INTRODUCTION
Vitiligo is an acquired, chronic, multifactorial depigmenting disorder of the skin. It affects approximately 0.5% of the general population and is characterized by white macules resulting from selective loss of epidermal melanocytes.[1,2] The primary defect is related to defective melanocyte regeneration and/or proliferation.[3] The disease shows no sex predilection and affects both sexes equally.[4]
Stability is one of the most important parameters in the management of vitiligo. It is broadly classified into two subtypes, namely stable and unstable vitiligo.[5] The Indian Association of Dermatologists, Venereologists, and Leprologists (IADVL) defines stability as “absence of new lesions, lack of progression of existing lesions, and absence of Koebner phenomenon during the last 1 year.”[6] Vitiligo activity is a prominent step during the development or extension of disease as vitiligo is characterized by episodes of stability and instability.[7]
At the progressing edge of the depigmented areas, the inflammatory reaction leads to the destruction of melanocytes, thereby indicating activity of the disease.[8] Available literature points toward an elevated ratio of CD8/CD4-positive T cells in the margins of active vitiligo lesions. There is also evidence of activated dendritic melanocytes.[9] Attack on epidermal keratinocytes by inflammatory infiltrate may be considered a diagnostic clue for disease activity.[10]
At present, the inflammatory infiltrates are not sufficiently characterized so as to completely define their role in determining instability of the disease. The current study aims to assess the relative number and distribution of T-lymphocytes, B-lymphocytes, macrophages, and Langerhans cells (LCs) in cases of unstable vitiligo.
MATERIALS AND METHODS
An observational cross-sectional study was carried out in the Department of Pathology and Dermatology of an Academic Medical Center from 2017 to 2020. Informed consent was taken from all patients who participated in the study. The study was duly approved by the Institutional Ethics Committee.
Patients with history and clinical features suggesting progression of the existing lesions and/or appearance of new lesions, presence of new-onset Koebner phenomena, newly diagnosed cases, or patients who stopped treatment for the past 3 months were considered unstable vitiligo cases as per IADVL guidelines. These cases were included in the study. Cases of segmental vitiligo and those on therapy were excluded.
A 3-mm punch biopsy was taken from two sites: Lesional and perilesional area. In cases of multiple macules, a freshly appeared macule or a macule that showed a recent increase in size was selected for biopsy. The biopsy was kept in 10% neutral buffered formalin and sent for further processing. Histopathological evaluation was done with respect to five parameters: Epidermal spongiosis, epidermal lymphocytes, basal cell vacuolation, dermal melanophages, and dermal lymphocytes.[5]
Immunohistochemistry for CD3, CD20, CD68, and CD1a was performed on both lesional and perilesional skin biopsies to evaluate the distribution and number of T-lymphocytes, B-lymphocytes, macrophages, and LCs, respectively. The cells were counted in 5 high-power fields (×40). Melan-A immunohistochemistry was performed to identify melanocytes.
The histological and immunohistochemical findings were correlated with clinical findings. Statistical analysis was done using Spearman’s rank coefficient correlation test to check the correlation between histological parameters and scoring in lesional biopsy whereas paired t-test was used to compare inflammatory cells in lesional and perilesional skin. To study multiple variables, multiple logistic regression was performed.
RESULTS
Clinical parameters
A total of 30 cases were included in the study. The age of presentation ranged from 6 years to 65 years (mean – 26.1 years). There were 11 males (36.67%) and 19 females (63.33%). M: F ratio was 1:1.7. No significant difference was noted in age distribution between males and females by Chi-square test (P < 0.284). Seven patients (23%) had a positive family history of vitiligo. Koebner’s phenomenon was seen in 22 (73.3%) patients.
The patients were categorized into focal or generalized vitiligo. The study included five cases of focal vitiligo and 25 cases of generalized vitiligo. The most common sites of skin biopsy were the upper extremity (43.3%) followed by the lower extremity (30%) and chest (10%) [Figure 1a-c].
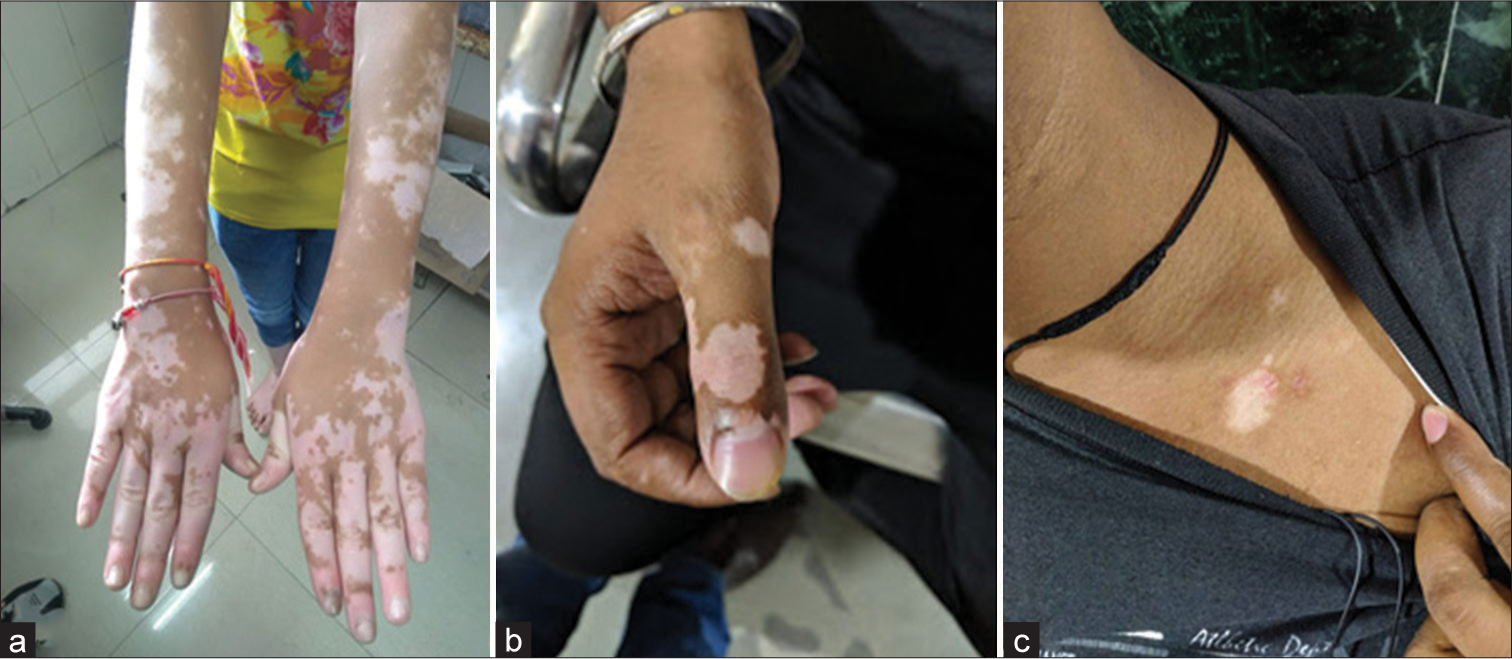
- (a-c) Common sites of lesion upper extremity and chest.
The histopathological parameters [Table 1] observed in each case are shown in Figure 2a-d. The presence of individual parameters was awarded a score of 1 whereas the absence was awarded 0. The scores in lesional skin biopsy ranged between 0 and 4 with 3 as the most common score which was seen in 12 (40%) patients. Twenty patients (66.6%) scored more than 2 and were classified histologically as unstable cases whereas 10 (33.33%) patients scored 2 or less and were classified as stable cases. Distribution of scores in perilesional skin ranged from 0 to 3 with 2 being the most common score which was seen in 12/30 (40%) cases [Table 2]. The presence of epidermal lymphocytes correlated best with scoring with a correlation coefficient of 0.721 (P < 0.05). Dermal lymphocytes >100 also correlated with scoring with a correlation coefficient of 0.71 (P < 0.05). Multiple logistic regression showed epidermal lymphocytes (odds ratio [OR] = 5.34, confidence interval [CI] = 2.24–14.26, P = 0.002) and dermal lymphocytes >100 (OR = 3.37, CI = 1.24–8.26, P = 0.007). The rest of the parameters were found to be non-significant.
| S. No. | Histological feature | Lesional n(%) |
|---|---|---|
| 1. | Spongiosis | 02 (6.67) |
| 2. | Epidermal lymphocytes | 18 (73.3) |
| 3. | Basal cell vacuolation | 29 (96.6) |
| 4. | Dermal lymphocytes >100/5 hpf | 08 (60) |
| 5. | Melanophages | 27 (90) |
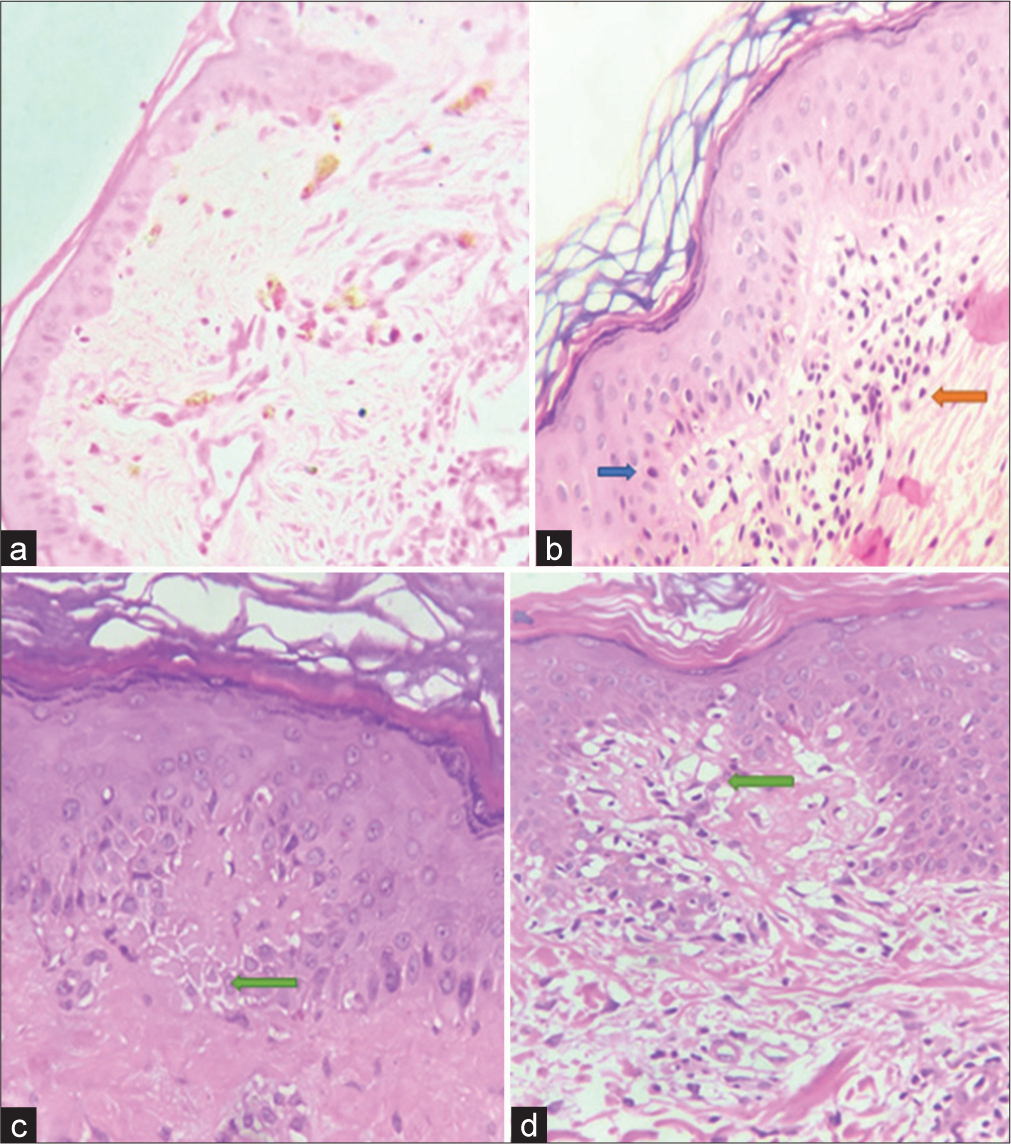
- Histopathological parameters (a) Dermal melanophages, (b) Epidermal lymphocytes (blue solid arrow) and dermal lymphocytes (orange solid arrow), (c) Spongiosis (green solid arrow), and (d) Basal cell vacuolation (green solid arrow).
| S. No. | Diagnosis | Total score | Lesional n(%) | Perilesional n(%) |
|---|---|---|---|---|
| 1. | Unstable vitiligo | 5 | 0 | 0 |
| 2. | Unstable vitiligo | 4 | 8 (26.6) | 0 |
| 3. | Favors unstable, clinical correlation required | 3 | 12 (40) | 09 (30) |
| 4. | Favors stable, clinical correlation required | 2 | 7 (23.3) | 12 (40) |
| 5. | Strongly favors stable vitiligo, clinical correlation essential | 0–1 | 3 (10) | 09 (30) |
| Median score | 3 | 2 |
Immunohistochemical evaluation of number of cells
T cells
CD3-positive T cells count in lesional skin ranged from 4 cells/5 hpf to 112 cells/5 hpf. The mean T cell count in the lesional skin was found to be statistically significant (P < 0.05), thereby showing that the count of T cells in lesional skin is significantly higher than in perilesional skin [Table 3]. The cell count correlated with an increase in vitiligo histological score [Figure 3a].
| Cases | CD3 Mean±SD | CD68 Mean±SD | CD1a Mean±SD | P-value | Correlation with vitiligo score P-value | |
|---|---|---|---|---|---|---|
| Lesional | 30 | 50.9±31.62 | 12.87±6.22 | 58.31±22.57 | <0.0001 | <0.0001 |
| Perilesional | 30 | 12.23±6.31 | 5.13±3.36 | 22.83±12.44 |
SD: Standard deviation, CD: Cluster of differentiation
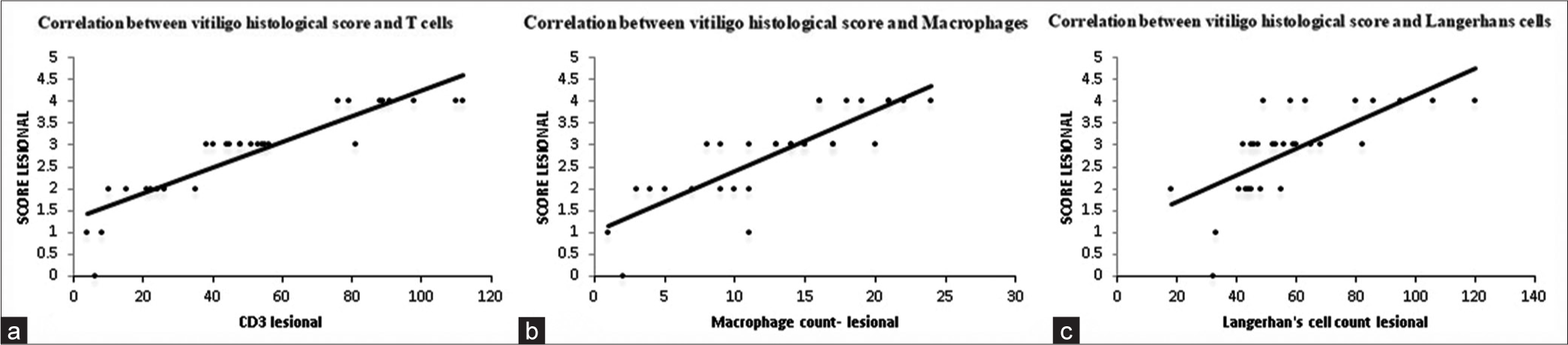
- Correlation between vitiligo histological score and numbers of (a) T cells, (b) Macrophages, and (c) Langerhans cells. CD: Cluster of differentiation
B cells
The B cell counts ranged from 0 to 4 cells/5 hpf with a mean of 1.4 cells/5 hpf and in perilesional skin ranged between 0 and 2 cells/5 hpf with a mean of 0.87 cells/5 hpf. B cell count both in the lesional and perilesional skin was too low for any statistical analysis. They were found to be distributed only in the dermis.
Macrophages
Macrophages or CD68-positive cell count in lesional skin ranged between 3 and 25 cells/5 hpf. The means of macrophage counts in lesional and perilesional skin were compared using the paired t-test. The difference between the means came out to be statistically significant (P < 0.05) [Table 3]. Macrophage count in the lesional skin was also correlated with the vitiligo histological score. An increase in macrophage count correlated with the rise in score [Figure 3b].
LCs
LCs were counted in the epidermis of the skin in five high-power fields in 29 cases. In lesional skin, the count ranged between 18 and 120 cells/5hpf whereas it ranged from 6 to 51 cells/5 hpf in perilesional skin. The difference between the means came out to be statistically significant (P < 0.05). Hence, the count in lesional skin was significantly higher than in perilesional skin [Table 3]. LC count in the lesional skin was also correlated with the histological score [Figure 3c].
The correlation was studied among the various cells in lesional skin using the Spearman rank correlation coefficient. The correlation coefficient between LC count and T cell count was found to be 0.782, LC count and macrophage count 0.717, and T cell count and macrophage count 0.859. All these correlations were statistically significant (P < 0.05) [Table 4].
| Langerhans cells | T cells | Macrophages | |
|---|---|---|---|
| Langerhans cells | |||
| Correlation coefficient | - | 0.782 | 0.717 |
| P-value | <0.0001 | <0.0001 | |
| n | 29 | 29 | |
| T cells | |||
| Correlation coefficient | 0.782 | - | 0.859 |
| P-value | <0.0001 | <0.0001 | |
| n | 29 | 30 | |
| Macrophage | |||
| Correlation coefficient | 0.717 | 0.859 | - |
| P-value | <0.0001 | <0.0001 | |
| n | 29 | 30 | |
Multiple logistic regression analysis showed T cells (OR = 3.04, CI = 1.64–6.26, P = 0.003), macrophages (OR = 4.34, CI = 3.34–12.46, P = 0.001), and LCs (OR = 7.34, CI = 4.78–18.56, P = 0.002). B cells were non-significant.
Immunohistochemical evaluation of the distribution of cells
The distribution of cells was studied in only lesional skin due to the high activity of inflammatory cells in that area. In the epidermis, the distribution was studied in all five layers (stratum basale, stratum spinosum, stratum granulosum, stratum corneum, and stratum lucidum). In the dermis, the distribution of cells was studied in the following areas: Interstitium, hair follicles, adnexa, blood vessels, and nerves. Distribution was observed for T cells, B cells, and LCs. B cells were not studied as their number was very low.
T cells
T cells were found in both epidermis and dermis in 22 (73.33%) cases whereas they were found exclusively in the dermis in 8 (26.7%) cases. There was no case in which they were found only in the epidermis.
In the epidermis, T cells were found exclusively in stratum basale in 12 (54.55%). They were seen in the dermis in all the cases. In all cases, they were present in the interstitium. In 43.3% of cases, T cells were found in the perivascular region and interstitium [Figure 4a-d].
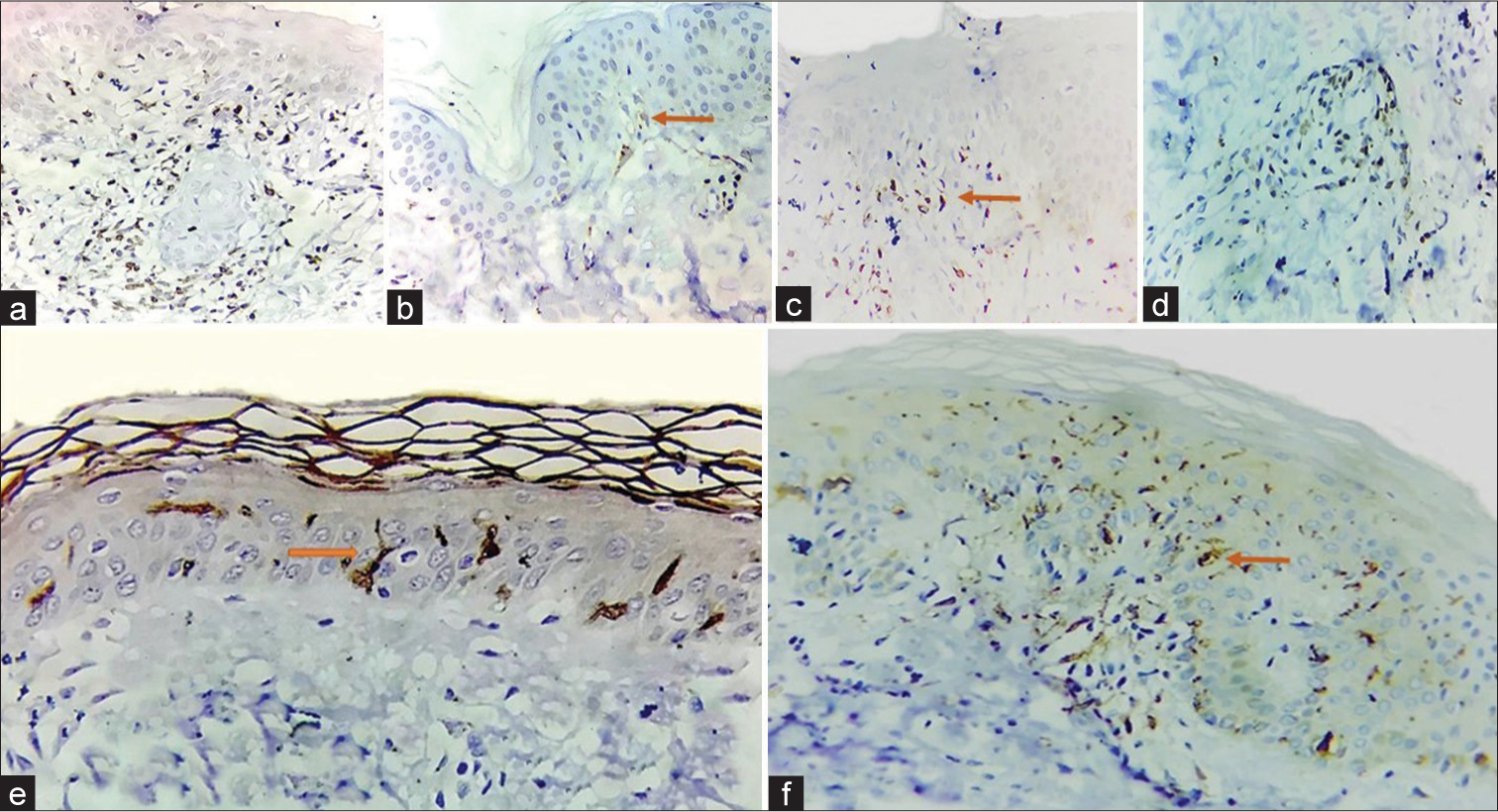
- Distribution of inflammatory cells (a) T cells in stratum basale, (b) T cells in stratum basale (solid orange arrow), (c) T cells in the dermis (orange solid arrow) (d) Perivascular T cells, (e and f) Intra-epidermal Langerhans cells (solid orange arrows).
Macrophages
They were observed in the dermis only in all 30 cases with no case showing intra-epidermal macrophages. Within the dermis, they were seen predominantly in the interstitium (86.67%).
LCs
LCs were seen exclusively in the epidermis in 24/30 (80%) cases. While in 5/30 (16.67%) cases, they were observed to be present in both the epidermis and dermis [Figure 4e and f]. In one case, they were not observed.
Melan-A immunohistochemistry
Melan-A immunohistochemistry showed the presence of melanocytes in the basal layer in the perilesional skin [Figure 5]. It was negative in lesional skin.
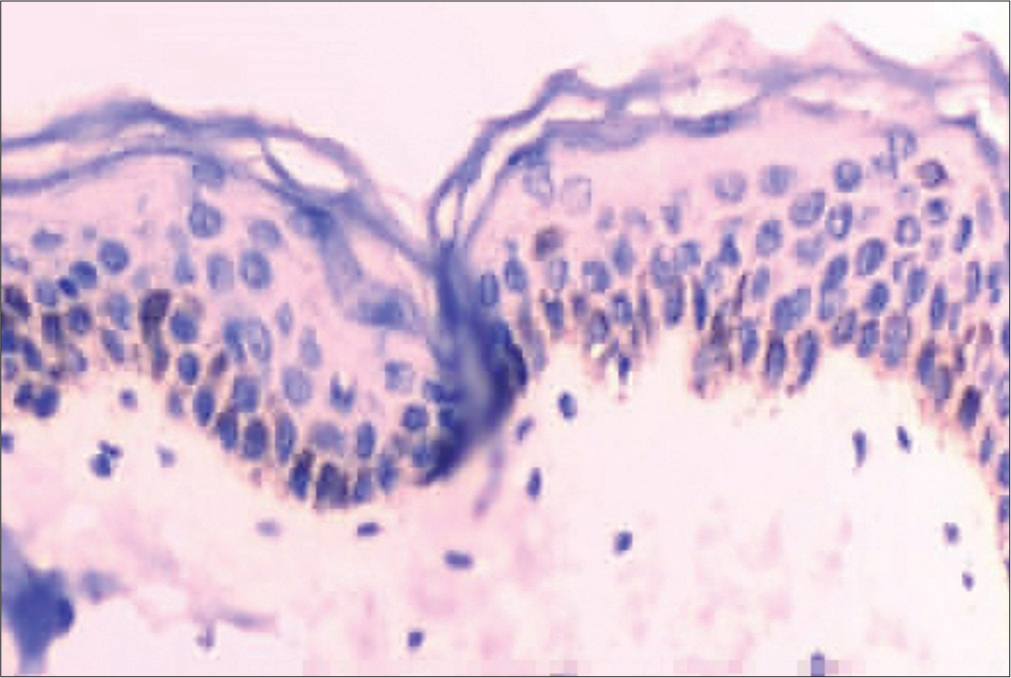
- Melan-A immunohistochemistry on perilesional skin shows positivity in melanocytes.
DISCUSSION
Vitiligo is an acquired skin-specific autoimmune disorder characterized by the loss of melanocytes in the epidermis which results in hypopigmented macules. The exact mechanism of the disease is largely unknown. Various theories have been proposed for its pathogenesis. At present, the most favored theory is the “Convergence theory” of vitiligo.[11] However, autoimmune-mediated destruction of melanocytes is a very popular theory because many studies show an increase in inflammatory infiltrates in the edge of actively progressing lesions and vitiligo is also known to coexist with many other autoimmune diseases.[12-17]
The cornerstone of management lies in the correct categorization of lesions into stable and unstable vitiligo which is done clinically. However, in the present study, histopathological parameters were evaluated, scored and recommended diagnosis given based on these parameters. Clinical and histological features along with immunohistochemistry for T cells, B cells, LCs, and macrophages were studied in 30 patients diagnosed clinically as unstable vitiligo based on IADVL guidelines.
M: F ratio was 0.57:1. Most of the patients fell in the age group of >20 years (40%). According to literature,[18,19] incidence of the disease is almost equal in males and females and most of the patients are within the age group 10–30. Our study showed similar findings. Higher presentation in females may be attributed to a small sample size.[20-22] Extremities (75%) were the most common site of unstable vitiligo. It can be explained as hands and feet are injury-prone areas.
Histological parameters were evaluated as per the scoring system given in a previous study by the authors.[5] Twenty cases (67%) were histologically categorized as unstable. However, in 10 cases (33%), there was discordance since they were classified as stable vitiligo histologically, but they were clinically unstable. The significance of this discordance needs to be studied further in clinical studies with long-term follow-up.
The correlation of the five histological parameters with scoring was also studied in lesional skin. Epidermal lymphocytes showed the best correlation followed by dermal lymphocytes with correlation coefficients 0.72 (P < 0.05) and 0.71, respectively. Basal cell vacuolation was the most commonly observed histological parameter followed by melanophages while spongiosis was the least common parameter. The above findings are in concordance with a previous study done by Awad and Moftah.[10]
Immunohistochemical expression of CD3, CD20, CD68, and CD1a was studied in inflammatory infiltrate in lesional and perilesional skin. The expression of CD3, CD68, and CD1a was found to be high in lesional skin. There was a significant increase in T cells in the dermis in lesional skin as compared to perilesional skin. T cell number correlated well with histological scoring, i.e., a higher number of CD3-positive cells was correlated with higher scores in lesional skin. This finding is in concordance with other studies that demonstrated a high number of T cells in the lesional skin of vitiligo.[10,23-25] T-cells were found in highest numbers in basal layer of the epidermis while in dermis they were found predominantly in the interstitium and around the blood vessels. This was comparable to the findings observed by Le Poole et al.[12] where T cell distribution was seen mainly concentrated in the basal layer of epidermis.[8,14,26] Another study conducted by Awad and Moftah found that all unstable lesions were positive for CD3 and CD68 and negative for CD20. However, quantification or further characterization was not done.[10]
A study by Benzekri et al. revealed infiltration of CD8-positive T lymphocytes in the epidermis and dermis with strong expression of E-cadherin.[27] Similarly, another study by Le Poole et al.[12] in perilesional skin biopsies from three patients found a marked T cell infiltrate in the dermis and basal layer of the epidermis with a high CD8 to CD4 ratio. CD36 showed an increase in the dermal macrophage counts. LCs were found to be increased in the perilesional skin. However, this finding was found to be inconsistent, and the significance was not known. They concluded that the presence of T cells and macrophages in vitiliginous skin parallels melanocyte disappearance.[14]
There are two major mechanisms by which T cells damage melanocytes: (i) Cytokine and (ii) cytotoxic T-cell-mediated killing. These cells travel from the dermal vasculature to the epidermis based on signaling and cause damage. In unstable vitiligo, CD8-positive T cells lead to the death of melanocytes due to chemokine-dependent recruitment. Activated CD8-positive T cells express interferon gamma which further recruits CD8-positive T cells to the skin thereby leading to the destruction of melanocytes.[28] Our study showed a high number of T cells in lesional skin with an increased number in basal layers of the epidermis and interstitium and blood vessels in the dermis supporting the pathogenesis.
We also found a higher number of melanophages in the dermis in lesional skin (mean = 12.87/5 hpf). This rise was found to be significant (P < 0.05) and correlated well with the histological score (correlation coefficient 0.8). The findings are consistent with a previous study by Awad and Moftah.[10] The increase is possibly due to the engulfment of pigment by dermal histiocytes which occurs due to vacuolar degeneration of basal cells while moving upward toward the upper layers of epidermis.[10] B cells were also found to be present in the dermis but their numbers were too low for any further analysis.
There are contradicting studies for LCs in vitiligo. Hatchome et al. studied 31 cases of vitiligo for possible functional impairment of LCs and found that the number of LCs was not significantly different in cases and controls. They postulated that it is not the number but there is a functional impairment of LCs in vitiligo.[29]
In another study by Hatchome et al.,[29] 26 cases of nonsegmental vitiligo were studied for the presence of LCs in the epidermis. They found that depletion of LCs happens in patients with active and re-pigmenting non-segmental type vitiligo. They suggested that depletion in depigmented lesions is due to the migration of LCs to lymph nodes to present certain antigens. They further suggested that repopulated epidermal LCs may play a role in inhibiting the proliferation of epidermal melanocytes in depigmenting lesions. Similarly, another study by Shoeib et al.[30] found a reduction in the number of LCs in patients of vitiligo before treatment with narrow-band ultraviolet B as compared to normal skin and suggested that reduction is directly proportional to disease activity and destruction of melanocytes.
Few other studies revealed increased epidermal LCs in vitiligo patients and an increase in populations of CD11-positive myeloid dermal dendritic cells and CD207-positive LCs in vitiligo, suggesting the capability of LCs to activate the dendritic cells. The current view is that LCs instruct T lymphocytes to mount effector responses when stimulated by a signal. The epidermal microenvironment is important for the programming of LC biology. They present antigens to cytotoxic T cells and herein may lie their importance in vitiligo. In our study, the LC count in lesional skin (mean = 58.31/5 hpf) was found to be higher and statistically significant (P < 0.05). The number also correlated with the scoring (correlation coefficient = 0.7). They were found to be distributed mainly in the epidermis which is similar to other studies.
CONCLUSIONS
The findings of the present study may not lead to any immediate changes in the management of vitiligo. However, it may have important implications in the future. Further work in the future will define the exact role of these cells in the pathogenesis of vitiligo along with the specific receptors and molecules taking part in this mechanism. This will help in designing novel targeted therapy modalities in the future. The increase in T cells, macrophages, and LCs was found to be associated with instability in lesions of vitiligo. This study highlights the importance of biopsy and immunohistochemistry as additional tools in the clinical assessment of stability in vitiligo.
Ethical approval
The research/study was approved by the Institutional Review Board at Safdarjung Hospital, approval number IEC/VMMC/SJH/Thesis/October/2017-169, dated 19-02-2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- A review and a new hypothesis for non-immunological pathogenetic mechanisms in vitiligo. Pigment Cell Res. 2006;19:406-11.
- [CrossRef] [PubMed] [Google Scholar]
- Vitiligo: Focus on clinical aspects, immunopathogenesis, and therapy. Clinic Rev Allergy Immunol. 2018;54:52-67.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathologic analysis of stable and unstable vitiligo: A study of 66 cases. Am J Dermatopathol. 2016;38:608-13.
- [CrossRef] [PubMed] [Google Scholar]
- IADVL dermatosurgery task force. Standard guidelines of care for vitiligo surgery. Indian J Dermatol Venereol Leprol. 2008;74:S37-45.
- [Google Scholar]
- Stability in vitiligo: Is there a perfect way to predict it? J Cutan Aesthet Surg. 2013;6:75-82.
- [CrossRef] [PubMed] [Google Scholar]
- Inflammatory changes in vitiligo: Stage I and stage II depigmentation. Am J Dermatopathol. 2004;26:108-12.
- [CrossRef] [PubMed] [Google Scholar]
- CD8+ T cells from vitiligo perilesional margins induce autologous melanocyte apoptosis. Mol Med Rep. 2013;7:237-41.
- [CrossRef] [PubMed] [Google Scholar]
- Activity status in vitiligo lesions: Diagnostic clues. J Egypt Womens Dermatol Soc. 2014;11:55-61.
- [CrossRef] [Google Scholar]
- Review of the etiopathomechanism of vitiligo: A convergence theory. Exp Dermatol. 1993;2:145-53.
- [CrossRef] [PubMed] [Google Scholar]
- Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol. 1996;148:1219-28.
- [Google Scholar]
- Innate immune mechanisms in vitiligo: Danger from within. Curr Opin Immunol. 2013;25:676-82.
- [CrossRef] [PubMed] [Google Scholar]
- Coexistence of Langerhans cells activation and immune cells infiltration in progressive nonsegmental vitiligo. J Dermatol Sci. 2014;73:83-5.
- [CrossRef] [PubMed] [Google Scholar]
- Th17 cells and activated dendritic cells are increased in vitiligo lesions. PLoS One. 2011;6:e18907.
- [CrossRef] [PubMed] [Google Scholar]
- Type I interferon signature in the initiation of the immune response in vitiligo. Pigment Cell Melanoma Res. 2014;27:398-407.
- [CrossRef] [PubMed] [Google Scholar]
- Vitiligo: Compendium of clinicoepidemiological features. Indian J Dermatol Venereol Leprol. 2007;73:149-56.
- [CrossRef] [PubMed] [Google Scholar]
- Studies on vitiligo. I. Epidemiological profile in Calcutta, India. Genet Epidemiol. 1985;2:71-8.
- [CrossRef] [PubMed] [Google Scholar]
- Vitiligo: A comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-91.
- [CrossRef] [PubMed] [Google Scholar]
- Pre-vs. post-pubertal onset of vitiligo: Multivariate analysis indicates atopic diathesis association in pre-pubertal onset vitiligo. Br J Dermatol. 2012;167:490-5.
- [CrossRef] [PubMed] [Google Scholar]
- Childhood-and later-onset vitiligo have diverse epidemiologic and clinical characteristics. J Am Acad Dermatol. 2012;66:954-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical markers of vitiligo activity. J Am Acad Dermatol. 2017;76:856-62.
- [CrossRef] [PubMed] [Google Scholar]
- An immunohistological study of cutaneous lymphocytes in vitiligo. J Pathol. 1993;170:149-55.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical studies from vitiligo: Comparison between active and inactive lesions. Yonsei Med J. 1994;35:404-10.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features and histological findings are potential indicators of activity in lesions of common vitiligo. Br J Dermatol. 2013;168:265-71.
- [CrossRef] [PubMed] [Google Scholar]
- The role of memory CD8+ T cells in vitiligo. J Immunol. 2019;203:11-9.
- [CrossRef] [PubMed] [Google Scholar]
- Possible functional impairment of Langerhans' cells in vitiliginous skin. Reduced ability to elicit dinitrochlorobenzene contact sensitivity reaction and decreased stimulatory effect in the allogeneic mixed skin cell lymphocyte culture reaction. Arch Dermatol. 1987;123:51-4.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the Langerhans cells role in vitiligo and its relationship to NBUVB. J Cosmet Dermatol. 2021;20:3642-8.
- [CrossRef] [PubMed] [Google Scholar]






