Translate this page into:
Microbial Flora in Chronic Periodontitis: Study at a Tertiary Health Care Center from North Karnataka
Address for correspondence: Dr. Kirtilaxmi K. Benachinmardi, E-mail: drkeertilaxmikb@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background and Objective:
Periodontitis is a major public health problem in India with a prevalence of 60–80%. If untreated it acts as a risk factor for systemic diseases. Data on anaerobic periodontal microflora in the Indian population is very scarce. Hence, this study was undertaken to know the nature of oral microbiota in chronic periodontitis in this region of India and also the semiquantitative study in pre- and post-treatment group and to determine antibiotic susceptibility pattern for aerobic isolates.
Materials and Methods:
The present study was conducted on 60 cases. Material was collected from the subgingival pockets in patients with chronic periodontitis attending the Periodontology, Outpatient Department. Clinical samples were transported to the laboratory in fluid thioglycollate medium. Initially Gram's stain and Fontana stains were done. Aerobic, anaerobic, and microaerophilic culture were put up. Antibiotic sensitivity test was done for aerobic isolates.
Results:
Sixty samples yielded 121 isolates of which 78.34% were polymicrobial, 11.66% were monomicrobial and oral commensals were grown in 10% cases. Out of 121 isolates 91.74% were anaerobic, 7.43% were aerobic and 0.83% were microaerophilic. Fusobacterium species was the most common isolate among anaerobes. Using “paired t-test” “P” value was significant indicating significant reduction in colony count after phase-I periodontal therapy.
Conclusion:
This study has shown that anaerobic bacteria are important cause of chronic periodontitis, along with aerobes and microaerophilic organisms. Fusobacterium spp, Bacteroides fragilis, Porphyromonas spp and Prevotella intermedia are the most common anaerobic pathogens. Bacterial culture methods are still economical and gold standard.
Keywords
Aerobe
anaerobe
oral microbial flora
periodontitis
subgingival plaque
INTRODUCTION
Symbiosis is an intimate association between the host and one or more colonizing bacteria. Human beings are colonized with an enormous number of bacteria; by-and-large skin, gut, urogenital tract and oral cavity are the typical sites. However, oral cavity is the one body site where co-operativity between the bacteria and host breaks down. If proper care is not taken to remove the plaque bacteria, mouth is prone to very common diseases like caries and periodontitis.[1]
Periodontitis is defined as an inflammatory disease of the supporting tissues of the teeth caused by specific microorganisms or group of specific microorganisms resulting in progressive destruction of periodontal ligament and alveolar bone with pocket formation, recession or both.[2] The disease prevalence is 13–57% worldwide and 65–80% in India accounting to a major health problem.[34]
Bacterial DNA sequencing study has suggested that nearly 19,000 bacterial phylotypes exist in the oral cavity. Nearly, 500 bacterial strains have been recovered from subgingival plaque, but only a small number are potential pathogens.[5]
Immunological, DNA probe assessment, bacterial enzyme detection and culture are the methods available for isolation of oral bacteria, among which culture remains economical and gold standard.[6]
There are good number of studies available from western world regarding the microbiota of oral cavity both in normal and in periodontitis condition, but data on anaerobic periodontal microflora in the Indian population is very scarce. Hence, the study was undertaken to isolate and identify the predominant aerobic and anaerobic microflora associated with adult generalized periodontitis, semiquantitative study of isolated microflora of adult generalized periodontitis in pretreatment and posttreatment groups and to study the antibiotic sensitivity pattern of aerobic isolates in pretreatment group.
MATERIALS AND METHODS
This study was conducted in the Department of Microbiology at a Tertiary Care Teaching Hospital. Samples were collected from subgingival pockets in patients with chronic periodontitis attending the Periodontology Outpatient Department at our Institute of Dental Sciences, over a period of 1-year from January to December 2010. The study comprised of 60 cases (Sample size was calculated using formula 4pq/d2 where p is prevalence, q is 100-p and d is 10% of the error).
Clinically diagnosed all new cases of chronic periodontitis were included in the study and patients with history of systemic conditions such as diabetes mellitus, nutritional deficiencies, pregnant woman, antibiotic usage in the last 3 months and patients with history of undergoing any dental procedures in the last 3 months were excluded. Samples were collected twice that is, in pretreatment and post-treatment periods after phase I therapy (scaling and root planning [SRP] along with amoxicillin and metronidazole for 1-week).
Sample collection
Tooth surfaces were dried with sterile gauze to avoid contamination by saliva. Sub gingival plaque sample was collected from most pathological site using sterile periodontal Gracy curette and placed in fluid thioglycollate medium in a test tube and brought to the microbiology laboratory and processed immediately.
Laboratory methods
In the laboratory sample in fluid thioglycollate medium was transferred to 1 ml of trypticase soya broth and vortexing was done for 1 min. Direct smears were prepared from the samples and Hucker's modification of Gram stain for anaerobes and modified Fontana staining methods were followed.[78] With a standard loop, a loopful of sample was taken from vortexed solution and inoculated onto 5% sheep blood agar (SBA), Macconkey agar (MA), brain heart infusion agar and blood agar supplemented with haemin and Vitamin K, Kanamycin Vancomycin laked blood agar (KVLB) and Bacteroid Bile Esculin Agar (BBE). Lawn culture was done and the plates were incubated at 37°C for 24 h aerobically (MA, SBA), under 5% CO2 (BA and BHI) and anaerobically (BBA supplemented with Vitamin K and hemin, KVLB, BBE). All aerobic isolates were identified and characterized biochemically by standard procedures as described by Mackie and McCartney practical medical microbiology.[9]
Anaerobic organism isolation
Semi-quantitative estimation of organisms was done by counting the number of colonies and taking into consideration of significant number of colonies (>50 colonies) and then subculturing on BBA. From the purity BA plate colony morphology, pigment, hemolysis, fluorescence and pitting were recorded and Gram stain (modified for anaerobic), spot indole test and catalase tests were performed. Further identification was made using sodium polynethenol sulfonate disc, nitrate disc, kanamycin (1 mg), vancomycin (5 μg) and colistin (10 μg) discs. The method used for obtaining anaerobiosis in the jar was “internal gas generating system” described by Vaidhyalingam and Laxminarayana.[10]
Antibiotic sensitivity testing
The antimicrobial susceptibility testing was done for aerobic isolates by disc diffusion method as described by Kirby and Bauer on Mueller-Hinton agar.[9]
Statistical analysis
Statistical analysis was performed using “paired t-test” comparing two groups.
RESULTS
A total of 120 samples (60 pre-treatment and 60 post-treatment) were collected from 60 patients. Our cases ranged between 22 and 70 years, maximum number of cases (22) were in the age group of 38–47 years and least (1) in the age group of 68–77 years. Of 60 cases, 33 (55%) were female and 27 (45%) were male. Sixty samples yielded 121 isolates of which 47 (78.34%) were polymicrobial, 7 (11.66%) were monomicrobial and oral commensals were grown in 6 (10%) cases.
Out of 47 polymicrobial isolates 27 (57.45%) were combination of 2 isolates and 20 (42.55%) were combination of 3 isolates. Out of 121 isolates 111 (91.74%) were anaerobic, 9 (7.43%) were aerobic and 01 (0.83%) was microaerophilic.
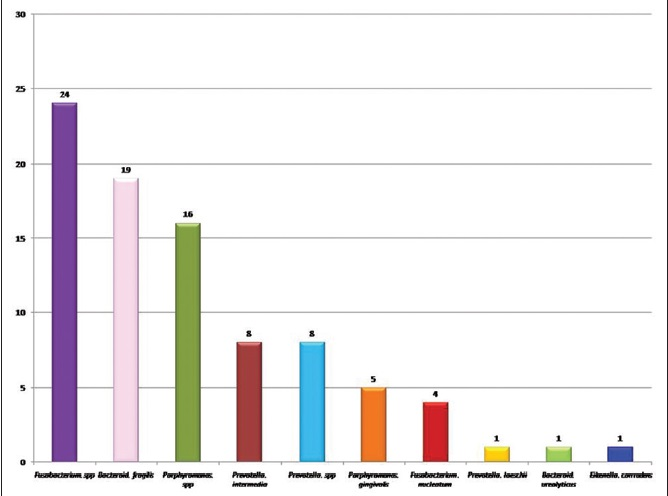
Out of 111 anaerobic isolates 87 (78.4%) were Gram-negative bacilli (GNB) and 24 (21.6%) were Gram-positive cocci (GPC). Among GNB Fusobacterium species was the most common isolate [Figure 1]. Out of 24 GPC, Micromonas micros were the most common isolate 17 (70.83%), followed by Peptostreptococcus anaerobius 4 (16.67%), Schleiferella asaccharolytica 2 (8.33) and Streptococcus mutans 1 (4.17%).
- Anaerobic Gram-negative bacilli
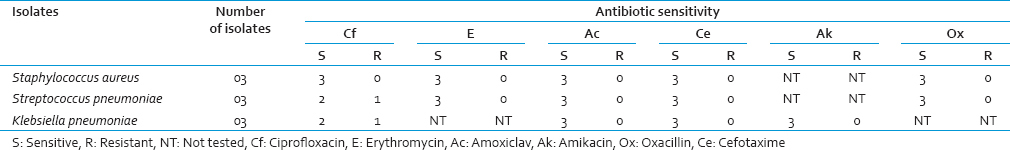
Aerobic isolates were Staphylococcus aureus, Streptococcus pneumoniae and Klebsiella pneumoniae three each. The antibiotic susceptibility pattern of aerobic isolates is shown in Table 1. The only microaerophilic organism isolated was Aggregatibacter actinomycetemcomitans [Figure 2]. Spirochetes (Treponema denticola) were observed in 19 (31.66%) cases by modified Fontana staining from direct plaque sample smears. The result of semiquantitative study is shown in Table 2.

- Low power microscopic picture of Aggregatibacter actinomycetemcomitans showing central star shaped colony
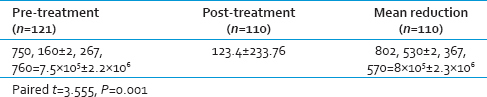
Using “paired t-test,” “p” value was significant indicating significant reduction in colony count after phase-I periodontal therapy. Paired t = 3.555, P = 0.001.
DISCUSSION
The search for the periodontal pathogens has been underway for more than 100 years and continues up today. The three factors which will determine the occurrence of active periodontal disease are a susceptible host, presence of pathogenic species and absence of so-called beneficial bacteria.[11] The goal of the present study was to attempt to understand the nature of microbial complexes that exist in subgingival plaque.
In the present study, the cases ranged from 20 to 70 years with a maximum number of cases in the age group of 38–47 years. In contrast, Sixou et al.[12] observed periodontitis cases in age group of 18–52 years, Antony et al.[13] in the age group of 30–60 years, Kamma et al.[14] in the age group of 14–35 years. It is a good old concept in the literature that the prevalence of periodontitis increases as age increases because of loss of periodontal tissue support, however maximum number of cases in our study were in adult group as observed by the previous authors.[15]
The male to female ratio in our study was 0.8:1. That is, females were more affected than males as observed in studies by Sixou et al.,[12] Salari and Kadkhoda[16] Daniluk et al.,[5] Nonnenmacher et al.[17] and Socransky et al.[6] In contrast in a study conducted by Sixou et al.[18] the ratio was M:F::1.3:1. However, there is no established, inherent difference between men versus women in their susceptibility to periodontitis.[15] 78.34% of our isolates were polymicrobial in nature in comparison to 11.06% of monomicrobial isolates. Our observations are consistent with the literature.[412161719] Polymicrobial nature of periodontal plaque has been well established. In the studies conducted by Sixou et al.[12] Nonenmacher et al.[17] Saini et al.[4] and Mane et al.[19] 97.14%, 100%, 72.28% and 97.3% of their isolates were polymicrobial, respectively. In contrast Salari and Kadkhoda[16] observed 18.28% of their isolates were polymicrobial.
Out of 78.34% of polymicrobial isolates 57.44% were a combination of two isolates, and 42.55% were a combination of three isolates. In a study conducted by Saini et al.[4] in India, they observed two isolates in 91.66% and three in 8.2%. In an another study conducted by Mane et al.[19] in India observed two isolates in 8%, three and four isolates 40% each and five isolates in 12%.
The results of the present study show the diversity of anaerobic bacteria in chronic periodontitis. Anaerobic bacteria were isolated in 91.74% of periodontitis cases. Various studies from India and other countries showed an isolation rate of strict anaerobes ranging from 42% to 100% in periodontitis cases.[4161920] In the two different studies conducted in France, detected 91.42% and 80.77% of anaerobic isolates. In a study by Mane et al.[19] 83% were anaerobes. Whereas studies conducted by Salari and Kadkhoda,[16] Daniluk et al.,[5] Nonnenmacher et al.[17] and Saini et al.[4] detected comparatively lower number of anaerobes that is, 41.22%, 57.1%, 53.84% and 64.25%, respectively. The varying recovery rates of isolation can be due to varying criteria of patient selection, culture method employed, geographical differences, and molecular technique used for identification.[419]
In the present study, Gram-negative anaerobes (78.4%) were predominantly isolated than the Gram-positive (21.6%) in the periodontitis cases. And our observations are comparable to the literature.[4561619] Fusobacterium spp (27.60%), Bacteroides fragilis (21.84%), Porphyromonas spp (24.48%), Prevotella intermedia (9.20%) [Figure 3], Fusobacterium nucleatum (4.60%) and Prevotella spp (9.20%) were the most common organisms isolated in our study. Other workers have isolated similar anaerobes, but in varying proportions. In an Indian study by Saini et al.[4] reported a much higher number of these isolates Porphyromonas spp 40%, P. intermedia 12%, Prevotella species 14%, Fusobactrium nucleatum 24% and Bacteroides 5%. Another Indian study by Mane et al.[19] reported higher number of P. intermedia (17.6%), F. nucleatum (41%), Fusobacterium spp (29.41%) were in accordance with our results. Sixou et al.[18] reported higher number of P. intermedia (15.78%) and F. nucleatum (7.01%). While Salari and Kadkhoda[16] and Nonnenmacher et al.[17] reported much lower number of these isolates. Comparison of spectrum of anaerobes in different studies is shown in Table 3. Beena et al.[21] concluded from their study on periodontal pathogen that, as the infection progress the proportion of anaerobes especially GNB like Prevotella, Porphyromonas and Fusobacterium increases.
- Prevotella intermedia

The Gram-positive anaerobic cocci constituted 21.6% of total anaerobic isolates in our study. Out of which M. micros (70.83%) were the most common isolates. Other GPC isolated in our study were P. anaerobius (16.67%), S. asachrolytica (8.33%) and S. mutans (4.17%).
Saini et al.[4] and Nonnenmacher et al.[17] reported M. micros in 23% and 51% respectively, which is much lower than our study. Sixou et al.[12] observed 52.94% of M. micros, 5.88% of P. anaerobius. In another study by Sixou et al.,[18] there were 14.28% each of M. micros and P. anaerobius. Salari and Kadkhoda[16] reported 2.9% and van Winkelhoff et al.[20] reported 70%, which is in accordance with our study. M. micros is considered to be a pathogen in the etiology of mixed anaerobic infections more often and in increased percentage from patients with periodontitis, especially in subjects with active disease.[2223242526] Our results are in accordance with the previous observation suggesting that M. micros may be associated with periodontitis.
In the present study group, aerobes constituted 7.43%, while Daniluk et al.[5] and Mane et al.[19] isolated 42.9% and 14% of aerobes, respectively. The aerobes isolated were S. aureus (33.33%), S. pneumoniae (33.33%) and K. pneumoniae (33.33%) and our results are in accordance with studies of Daniluk et al.[5] who reported Staphylococcus species (16.66%) and Streptococcus species (38.88%). Saini et al.[4] reported 42.85% of S. aureus and 44.44% of Klebsiella spp. They also reported Escherichia coli, Pseudomonas spp (14.28%) and nonfermenters (28.5%), which are not observed in our study.[45]
The only microaerophilic bacteria isolated in the present study is A. actinomycetemcomitans which accounts for 0.83% of total bacterial isolates. The isolation rate of A. actinomycetemcomitans from adult periodontitis was reported to be 53% by Antony et al.,[13] 9% by Nonnenmacher et al.,[17] 20.6% by Salari and Kadkhoda[16] and 14.6% by Saini et al.[4] These results show that A. actinomycetemcomitans seems to play a significant role in periodontitis along with the other anaerobes.
Spirochetes such as T. denticola were observed by modified Fontana staining in 31.66% of cases. In this study, there was significant decrease in bacterial count following SRP with P < 0.001, as observed in the literature. Two systemic reviews have suggested that adjuvant systemic antibiotic administration will have a better outcome than SRP alone.[2728] The predisposing factors for periodontitis include increasing age, prior history of periodontitis, smoking, stress, few drugs such as anticonvulsants, calcium channel blockers and oral contraceptive pills, systemic factors like HIV, Diabetes mellitus and hematological factors.[293031]
Periodontitis if untreated thought to act as a risk factor for systemic diseases like atherosclerosis, cardiovascular and cerebrovascular diseases, pregnancy complications, diabetes mellitus and others.[3132]
Since the concept of dental infection has been bacteriologically nonspecific and offers no rationale for antimicrobial treatment, careful assessment and isolation of both aerobes and anaerobes is the utmost importance in the treatment of orodental infection.[4]
CONCLUSION
This study has shown that anaerobic bacteria are important cause of chronic periodontitis, along with aerobes and microaerophilic organisms. Cultural methods are still economical and gold standard. Considering the scarce data on microbial flora in the Indian population, further studies for assessment of microbial profile in various forms of periodontitis should be carried out.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Classification of diseases and conditions affecting the periodontium. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA, eds. Carranza's Clinical Periodontology (10th ed). St. Louis Missouri: Elsevier; 2007. p. :103-4.
- [Google Scholar]
- Prevalence and distribution of principal periodontal pathogens worldwide. J Clin Periodontol. 2008;35:346-61.
- [Google Scholar]
- Aerobic and anaerobic bacteria in subgingival and supragingival plaques of adult patients with periodontal disease. Adv Med Sci. 2006;51(Suppl 1):81-5.
- [Google Scholar]
- Bailey and Scott's Diagnostic Microbiology. (11th ed). St. Louis, Missouri: Mosby, Inc. An Affiliate of Elsevier Science; 2002.
- [Google Scholar]
- A modified silver impregnation staining for leptospires. Indian Vet J. 1998;75:349-51.
- [Google Scholar]
- Mackie and McCartney Practical Medical Microbiology. (14th ed). Churchill Lvingstone: Elsevier; 2008.
- [Google Scholar]
- Internal gas generator system suitable for creating anaerobiosis. Indian J Surg. 1980;42:154-9.
- [Google Scholar]
- Microbiology of mandibular third molar pericoronitis: Incidence of beta-lactamase-producing bacteria. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:655-9.
- [Google Scholar]
- Actinobacillus actinomycetemcomitans and anaerobes in periodontotis. Indian J Med Microbiol. 1997;15:73-6.
- [Google Scholar]
- Predominant microflora of severe, moderate and minimal periodontal lesions in young adults with rapidly progressive periodontitis. J Periodontal Res. 1995;30:66-72.
- [Google Scholar]
- Chronic periodontitis. In: Lindhe J, Lang NP, Karring T, eds. Clinical Periodontology and Implant Dentistry Vol 1. (5th ed). Oxford: Blackwell Publishers; 2008. p. :398-495.
- [Google Scholar]
- Rate of cultivable subgingival periodontopathogenic bacteria in chronic periodontitis. J Oral Sci. 2004;46:157-61.
- [Google Scholar]
- Microbiological characteristics of subgingival microbiota in adult periodontitis, localized juvenile periodontitis and rapidly progressive periodontitis subjects. Clin Microbiol Infect. 2001;7:213-7.
- [Google Scholar]
- Evaluation of the mandibular third molar pericoronitis flora and its susceptibility to different antibiotics prescribed in france. J Clin Microbiol. 2003;41:5794-7.
- [Google Scholar]
- Porphyromonas gingivalis, Bacteroids forsythus and other periodontal pathogens in subjects with and without periodontal destruction. J Clin Periodontal. 2002;29:1023.
- [Google Scholar]
- Porphyromonas gingivalis, Bacteroids forsythus and other periodontal pathogens in subjects with and without periodontal destruction. J Clin Periodontal. 2002;29:1023.
- [Google Scholar]
- Microbial etiological agents of destructive periodontal diseases. In: Socransky SS, Haffajee AD, eds. Microbiology and Immunology of Periodontal Diseases. Oxford: Munksgaard, Blackwell; 1994. p. :78-111.
- [Google Scholar]
- The microflora of periodontal sites showing active destructive progression. J Clin Periodontol. 1991;18:729-39.
- [Google Scholar]
- Peptostreptococcus micros in human periodontitis. Oral Microbiol Immunol. 1992;7:1-6.
- [Google Scholar]
- Clinical, microbiological and immunological profile of healthy, gingivitis and putative active periodontal subjects. J Periodontal Res. 1996;31:195-204.
- [Google Scholar]
- Systemic anti-infective periodontal therapy. A systematic review. Ann Periodontol. 2003;8:115-81.
- [Google Scholar]
- A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol. 2002;29(Suppl 3):136-59.
- [Google Scholar]
- Chronic periodontitis. In: Newuman MG, Takei HH, Klokkevold PR, Carranza FA, eds. Carranza's Clinical Periodontology (10th ed). St. Louis Missouri: Elsevier; 2007. p. :497-99.
- [Google Scholar]
- Chronic periodontitis. In: Lindhe J, Laung NP, eds. Clinical Periodontology and Implant Dentistry (5th ed). Oxford: Blackwell Publishers; 2008. p. :424-6.
- [Google Scholar]
- Parameter on periodontitis associated with systemic conditions. American Academy of Periodontology. J Periodontol. 2000;71(5):876-9.
- [Google Scholar]
- Periodontal disease as a risk for systemic disease. In: Lindhe J, Lang NP, Karring T, eds. Clinical Periodontology and Implant Dentistry Vol 1. (5th ed). Oxford: Blackwell Publishers; 2008. p. :398-495.
- [Google Scholar]





