Translate this page into:
Performance of Xpert MTB/RIF on Ascitic Fluid Samples for Detection of Abdominal Tuberculosis
Address for correspondence: Prof. Sarman Singh, E-mail: sarman_singh@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Diagnosis of abdominal tuberculosis (TB) from ascitic fluid samples using routinely available diagnostic methods is challenging due to its paucibacillary nature. Although performance of Xpert MTB/RIF assay has been evaluated extensively on pulmonary samples, its performance on extrapulmonary samples is still under evaluation.
Objectives:
The objective of this study was to find out the performance of Xpert MTB/RIF on ascitic fluid samples obtained from suspected cases of abdominal TB. Performance was compared with Mycobacterium growth indicator tube-960 (MGIT-960) culture and in-house multiplex polymerase chain reaction (PCR). The latter detects and differentiates Mycobacterium tuberculosis and nontuberculous mycobacteria simultaneously.
Materials and Methods:
Sixty-seven patients suspected of probable/possible abdominal TB were included in this observational, prospective study. All samples were tested by Ziehl–Neelsen staining, MGIT-960 culture, in-house multiplex PCR, and Xpert MTB/RIF assay.
Results:
All 67 samples were smear negative. Seventeen (25.4%) were MGIT-960 culture positive while 12 (17.9%) were detected positive by the Xpert MTB/RIF assay and 9 (13.4%) by in-house multiplex PCR. Sensitivity and specificity of the Xpert MTB/RIF assay compared with the MGIT-960 culture were 70.6% (95%, confidence interval [CI]: 44.1–89.7) and 100% (95%, CI: 92.8–100) and that of in-house multiplex PCR were 52.9% (95%, CI: 30.9–73.8) and 100% (95%, CI: 92.8–100), respectively.
Conclusions:
Diagnostic yield of Xpert MTB/RIF assay on ascitic fluid samples was lower than MGIT-960 culture. We thus emphasize on the need for urgent discovery of new biomarkers for paucibacillary TB.
Keywords
Abdominal tuberculosis
ascitic fluid
Mycobacterium growth indicator tube-960 culture
multiplex polymerase chain reaction
Xpert MTB/RIF
INTRODUCTION
Tuberculosis (TB) remains a major health problem, with an estimated 9.6 million deaths every year.[1] Although in approximately 80% of cases this disease manifests as pulmonary TB (PTB), in the last few years, especially after HIV epidemic, incidence of extrapulmonary form of TB (EPTB) including abdominal TB has increased globally.[2] Studies show that a wide range (15–50%) of EPTB patients may have concomitant active pulmonary disease but will present with primary complaints pertaining only to abdominal TB.[345] In contrast, ascites can be a manifestation of various pathological conditions including noninfectious and infectious etiologies; TB is one of them, predominantly seen in high TB burden countries.[6]
The lack of explicit clinical features and ascertaining the primary cause of exudative collection, both delay diagnosis and treatment of the primary condition, lead to high mortality rate.[23] The standard diagnostic methods such as Ziehl–Neelsen (ZN) staining of smears and Lowenstein–Jensen (L-J) culture done from ascitic fluid are not very sensitive for the diagnosis of abdominal TB.[7] Liquid culture methods adopted by most countries in the last 10 years have improved the sensitivity to some extent but are also expensive and need sophisticated laboratory infrastructure.[89] Thus, for the diagnosis of EPTB, there is an urgent need for rapid new diagnostic methods.[10] Several nucleic acid amplification tests (NAATs), such as polymerase chain reaction (PCR), Gene Probe, and line probe, have been developed in the last few years claiming high sensitivity, specificity, and short turnaround time (TAT). These molecular tests have been developed using various insertion sequences, internal transcribed spacer (ITS) sequences, and other gene targets. Most of these PCR methods are aimed to detect Mycobacterium tuberculosis (MTB) while missing other Mycobacterium infections.[111213] For this purpose, our group and later on others have developed in-house multiplex PCR assays targeting genus-specific primers, MTB complex-specific primers, and also some commonly detected nontuberculous mycobacterial species.[111213] However, some in-house developed PCRs have shown region to region and laboratory to laboratory variations ranging from 18% to 99%, making them poorly acceptable.[141516171819] In 2010, Xpert MTB/RIF assay (Cepheid Inc., Sunnyvale, CA, USA) was endorsed by the World Health Organization (WHO), which detects MTB along with rifampicin (RIF) resistance within a shorter TAT of 2 h and has minimal risk of cross-contamination. Xpert MTB/RIF assay has extensively been used on pulmonary samples with pooled crude sensitivity and specificity of 92.5% and 98%, respectively.[20] However, evidence for using this assay for EPTB samples is weak.[182021] We recently reported poor performance of Xpert MTB/RIF assay on pleural fluid samples, which is another highly proteinous samples with high concentration of PCR inhibitors.[22] As such, there are very few studies reporting its usefulness on ascitic fluid and these too have used very small sample size, and to the best of our knowledge, there is no systemic study published from India.
The main aim of this prospective study was to evaluate the sensitivity and specificity of Xpert MTB/RIF and in-house multiplex PCR in comparison with phenotypic Mycobacterium growth indicator tube-960 (MGIT-960) liquid culture as standard. All surviving patients were followed up to 12 months irrespective of whether the laboratory results were positive or negative.
MATERIALS AND METHODS
Patients and samples
This prospective, observational study was carried out from January 2013 to December 2014 at the TB Laboratory, Division of Clinical Microbiology and Molecular Medicine, Department of Laboratory Medicine, All India Institute of Medical Sciences, New Delhi, which is an accredited (C&DST) laboratory. Samples from patients with clinical, radiological suspicion of abdominal TB were sent to our laboratory for microbiological confirmation. Another aliquot of the sample was simultaneously sent for cytopathological examination. Clinical symptoms mainly found among these patients were fever, weight loss, anorexia, vomiting, and distension of abdomen with or without recurrent diarrhea, cough, and abdominal pain. A total of 67 single nonrepetitive ascitic fluid samples (minimum 3 ml) were received from 67 patients.
Xpert MTB/RIF assay
One milliliter (ml) of ascitic fluid was tested by Xpert MTB/RIF assay following the manufacturer's protocol as reported previously.[23] Briefly, 1 ml of uncentrifuged ascitic fluid sample was lysed with 3 ml of SR buffer (3:1) and incubated for 15 min at room temperature. From the 3 ml mixture, 2 ml was transferred into Xpert MTB/RIF assay cartridges version G4 containing the wash buffer, reagents for DNA extraction and PCR amplification, fluorescent detection probes (five for the rpoB gene and one for internal control, Bacillus globigii spores). After proper mixing, the cartridge was loaded into Xpert MTB/RIF assay instrument. Results were generated in 2 h and reported as MTB negative or positive with semiquantified bacillary load as high, medium, intermediate, low, and RIF sensitive or resistant using software version 4.4a I (Cepheid Inc., Sunnyvale, CA, USA).
Sample processing
The remaining ascitic fluid sample was processed for smear, culture, and in-house multiplex PCR by N-acetyl-L-cysteine-sodium citrate-NaOH method as published elsewhere.[23] Processed samples were used for ZN staining for acid fast-Bacilli (AFB), MGIT-960 culture, and in-house multiplex PCR after DNA extraction.
Mycobacterium growth indicator tube-960 culture
Five hundred microliters of decontaminated sample was inoculated into the MGIT-960 culture tube containing 800 µl of OADC and PANTA supplement as per the manufacturer's instructions (Becton Dickinson Diagnostic Instrument Systems, Sparks, MD, USA). The inoculated MGIT tube was loaded in the automated MGIT-960 system, and the growth was continuously monitored by BD Epicentre®.
In-house multiplex polymerase chain reaction (mPCR)
DNA isolation for in-house mPCR was performed as mentioned previously. Briefly, 1 ml of decontaminated samples was lysed with lysozyme, followed by proteinaseK-SDS treatment. Proteins and macromolecules were precipitated using CTAB-NaCl solution. Nucleic acids were recovered from the aqueous phase after extraction with chloroform and iso-amyl alcohol, followed by precipitation with 70% ice cold ethanol. The precipitated DNA pellet was solubilized in 50 µL of tris-EDTA buffer, and 5 µl volume was used for in-house mPCR which was performed according to the previously published protocol.[182425] This in-house mPCR targets three genes: hsp-65 (genus-specific), esat-6 (MTB-specific), and ITS MAC region (specific for Mycobacterium avium complex). The sequences of primers were as follows:
-
hsp-65 F-ACCAACGATGGTGTGTCCAT
-
hsp-65 R-CTTGTCGAACCGCATACCCT
-
esat-6 F-GCGGATCCCATGACAGAGCAGCAG TGGA
-
esat-6 R-CCAAGCTTCCTATGCGAACAT CCCAGTGACG
-
ITS F-CCCTGAGACAACACTCGGTC
-
ITS R-ATTACACATTTCGATGAACGC.
The PCR was set up for 25 µL final volume and was run in a thermal cycler at the amplifying conditions of initial denaturation at 95°C for 10 min and 30 cycles of 95°C for 1 min, 59°C for 1 min, and 72°C for 1 min and a final extension of 72°C for 10 min. Amplified products were resolved through 2% agarose gel in tris-acetate buffer.
Statistical analysis
All the data of the Xpert MTB/RIF assay, MGIT-960, and in-house multiplex PCR were maintained on MS Excel 2007. Data were statistically analyzed to calculate sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
RESULTS
Patient details and clinical symptoms
Out of 67 patients, 43 (64.2%) were male and 24 (35.8%) were female, with a male to female ratio of 1:1.79. Their mean age was 41.0 ± 18.8 and 46.0 ± 20.4 for males and females, respectively. Majority (64 [95.5%]) of patients were adult cases and only 3 (4.4%) were children. Most patients presented with abdominal pain (64 [95.5%]), fever (54 [80.6%]), weight loss (45 [67.1%]), and headache (42 [62.7%]). Anorexia (42.5%) and vomiting (31.3%) were less common. On examination, all (100%) had abdominal dullness, 16 (23.8%) had abdominal tenderness, and 10 (14.9%) had hepatomegaly. Abdominal fluid collection was confirmed in all by abdominal ultrasonography. All patients were HIV negative and no patient was currently on antitubercular treatment (ATT). Chest radiograph abnormalities were noted in 34.4% (23/67) cases, which included pulmonary fibrosis in 10 (15%), pulmonary infiltrates in 7 (10.4%), pleural effusions in 4 (5.9%), and pleural thickening in 2 (3%).
Performance of smear and Mycobacterium growth indicator tube (MGIT)-960 culture
All ascitic fluid samples were smear negative for AFB. MGIT-960 culture was positive in 17 (25.4%) and negative in 50 (74.6%) cases. All cultures detected flagged positive by MGIT-960 system were identified as MTB by in-house mPCR [Table 1].

Performance of Xpert MTB/RIF
Sensitivity of Xpert MTB/RIF assay for detection of TB from ascitic fluid samples was determined by considering MGIT-960 culture as the gold standard. Out of 67 samples, Xpert MTB/RIF assay was positive in only 12 (17.9%) cases while 55 (82.1%) were negative. All 12 samples detected MTB by Xpert MTB/RIF were sensitive for RIF.
Performance of in-house mPCR
Of 67 samples tested by mPCR assay, only 9 (13.4%) were detected positive for genus as well as for MTB-specific targets and 58 (86.6%) were found negative for both genus- and species-specific PCR. No sample was positive for nontuberculous mycobacterial species. All these nine samples were also positive in MGIT-960 culture.
Comparison of MGIT-960 culture with Xpert MTB/RIF assay
The MGIT-960 culture was positive in 17 (25.4%) cases as highlighted above, and Xpert MTB/RIF assay was positive in 12 (70.5%) of these samples. No additional case could be detected by Xpert MTB/RIF [Figure 1]. The mPCR was positive in 9 (52.9%) of the 17 culture positive samples. Like Xpert MTB/RIF, the in-house mPCR also did not detect any additional case over the culture. The sensitivity and specificity of Xpert MTB/RIF and mPCR as compared to MGIT-960 culture are given in Table 1.
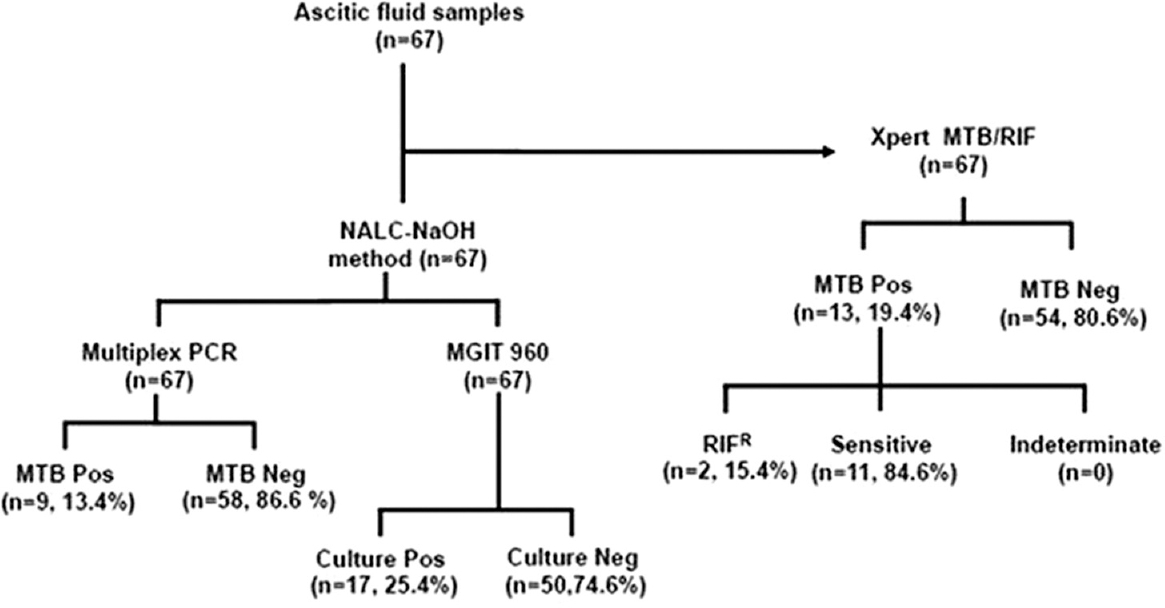
- Algorithm of the study with summary results of Xpert MTB/RIF, MGIT-960 and the mPCR
Initiation of antitubercular treatment (ATT) for tubercular ascites
Initiation of ATT was initiated in all 17 patients who were culture confirmed TB cases. Following the guidelines of standard care, as prescribed by the American Thoracic Society, treatment was also initiated in those patients who had strong suspicion of TB but no evidence of malignancy even if these were culture negative. The major criteria for suspecting TB were significant ascites with unexplained fever with or without weight loss, previous history of TB, contact with a TB patient, and radio-imaging signs of intestinal obstruction. It also included Mantoux test positivity, raised erythrocyte sedimentation rate, cytological findings, and ascitic fluid biochemistry. The other parameters (minor criteria) taken into consideration were white blood cell count in the ascitic fluid which widely ranged from <500 cells/mm3 to 1500 cells/mm3, adenosine deaminase >30 U/L, total protein >2.6 g/dl, and serum albumin gradient content <1.1 g/dl.
Follow-up of patients
All patients irrespective whether they were positive or negative for MTB were followed up for 12 months (9 months treatment plus 3 months posttreatment period). Follow-up was done to observe treatment outcome in confirmed TB cases and initiation of suggestive treatment outcome in probable/possible TB cases.
After follow-up, based on treatment status, three groups could be defined as confirmed TB cases (MGIT 960-culture/Xpert MTB/RIF/mPCR positive), possible TB (strong radiological/clinical/biochemical suspicion), and non-TB group diagnosed with diseases other than TB. Based on these criteria, of the 67 patients, 43 (64.2%) patients received ATT, of which 17 (39.5%) were confirmed TB cases and 26 (60.4%) cases were grouped as possible TB cases. Remaining 24 patients fulfilled the criteria of non-TB group [Table 2]. During follow-up of all patients, favorable treatment outcome was observed in all 17 (100%) confirmed TB cases. Interestingly, in possible TB cases where presumptive treatment for TB was started, favorable outcome was accomplished in 17/26 (65.4%) and unfavorable treatment outcome was seen in 9/26 (34.6%) cases. Hence, out of 67 patients, 34 (50.7%) patients got cured of TB and could be considered as true TB cases. Therefore, considering these 34 patients as actual TB cases, the overall diagnostic yield of three combined methods was 50% (17/34) only.

DISCUSSION
It is estimated that approximately 15–20% of TB cases are from extrapulmonary sites even though concrete data from high-burden countries such as India are lacking.[26] Most forms of EPTB but particularly the cases of tubercular ascites have remained diagnostic challenge over decades due to paucibacillary nature of ascitic fluid or the biopsy samples, thus leading to missed and delayed diagnosis.[927] Age-old conventional methods such as smear examination of ZN-stained smears provide extremely low sensitivity while L-J culture besides low yield takes 4–6 weeks to report growth results. Even though automated liquid culture systems have improved diagnostic yield and have also reduced time to culture positivity, these improvements are not to the desirable level. These limitations emphasize on urgent need for rapid diagnosis so that timely treatment can be started.[28] Newly introduced NAATs if used in combination with other tests provide improved diagnostic yield are now being widely used in high-resource settings, but high cost makes it complex to use these combinations in resource-limited setting.[29] In 2013, WHO endorsed use of Xpert MTB/RIF assay on pulmonary samples where it has been reported to be highly sensitive and specific[3031] and thus recommended for national TB programs in developing countries.[29] However, its utility for the diagnosis of EPTB particularly from ascitic fluid remains comparatively weak.[20]
We found that even in culture confirmed samples its yield was low (70.5%) indicating that in highly proteinous body fluids such as ascitic fluid, Xpert MTB/RIF-negative cases must be investigated further using other phenotypic methods. Previous studies have also reported low sensitivity of Xpert MTB/RIF on ascitic fluid samples; however, in these studies, the sample sizes were very small.[831323334] In the present study, the number of samples was significantly more. Most because of this reason, the diagnostic sensitivity of Xpert MTB/RIF assay was higher in our study than the earlier study done in South Africa, in which its sensitivity was 59% (95% CI, 39–69%) in culture confirmed ascetic fluid samples.[21] This difference could also be due to older version (G3) of the cartridges (not mentioned by the authors) in that study.[21] Nevertheless, Xpert MTB/RIF has shortest TAT (4 h) with high PPV. It means that if the Xpert MTB/RIF is positive, the ATT can be started without waiting for MGIT culture reports.
The sensitivity of in-house multiplex PCR was still poorer (52.9%) in comparison to culture, but it was equally specific as Xpert MTB/RIF. This in-house multiplex PCR has been found to be highly sensitive and specific in pulmonary samples with additional advantage of detecting nontuberculous mycobacteria simultaneously.[1819] However, poor performance of the PCR in ascitic fluids could be due to high concentration of PCR inhibitors.
CONCLUSION
Even though for detection of PTB liquid culture and molecular methods have shown outstanding sensitivity, in our study liquid culture and molecular tests such as Xpert MTB/RIF assay and in-house multiplex PCR evaluated for detection of abdominal TB from ascitic fluid samples showed poor sensitivity. However, automated MGIT-960 culture system provided slightly better yield as compared to molecular method. On the basis of these data, therefore, we strongly stress on finding new tools and discovery of new biomarkers for rapid diagnosis of abdominal TB.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank Mr. Vinod Kumar, Ms. Divya Sharma, and Ms. Nigam Kumari (all from the Department of Laboratory Medicine, AIIMS, New Delhi) for their technical help in this study.
REFERENCES
- 2015. Global Tuberculosis Report. Available from: http://www.apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1
- Diagnostic markers for tuberculosis ascites: A preliminary study. Biomark Insights. 2010;5:87-94.
- [Google Scholar]
- Systematic review: Tuberculous peritonitis – Presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005;22:685-700.
- [Google Scholar]
- Diagnostic dilemma of abdominal tuberculosis in non-HIV patients: An ongoing challenge for physicians. World J Gastroenterol. 2006;12:6371-5.
- [Google Scholar]
- Ascites: Diagnosis and management. 2000. J Indian Acad Clin Med. 5:81-9. Available from: http://www.ftp://60-241-92-16.static.tpgi.com.au/software/jact00i1p81.pdf
- [Google Scholar]
- Increase in extrapulmonary tuberculosis in England and Wales 1999-2006. Thorax. 2009;64:1090-5.
- [Google Scholar]
- Rapid diagnosis of pulmonary and extrapulmonary tuberculosis in HIV-infected patients. Comparison of LED fluorescent microscopy and the GeneXpert MTB/RIF assay in a district hospital in India. Tuberc Res Treat. 2012;2012:932862.
- [Google Scholar]
- Comparative proteomic analysis of sequential isolates of Mycobacterium tuberculosis from a patient with pulmonary tuberculosis turning from drug sensitive to multidrug resistant. Indian J Med Res. 2015;141:27-45.
- [Google Scholar]
- Analysis of LAM and 38kDa antibody levels for diagnosis of TB in a case-control study in West Africa. Int Sch Res Notices 2012:e237823.
- [Google Scholar]
- Characterization of a DNA probe for detection of Mycobacterium tuberculosis complex in clinical samples by polymerase chain reaction. J Clin Microbiol. 1992;30:2173-6.
- [Google Scholar]
- Detection of Mycobacterium tuberculosis DNA in clinical samples by using a simple lysis method and polymerase chain reaction. J Clin Microbiol. 1993;31:1019-21.
- [Google Scholar]
- A simple method for diagnosing M. tuberculosis infection in clinical samples using PCR. Mol Cell Probes. 1991;5:385-95.
- [Google Scholar]
- Evaluation of in-house mpt64 real-time PCR for rapid detection of Mycobacterium tuberculosis in pulmonary and extra-pulmonary specimens. Braz J Infect Dis. 2012;16:493-4.
- [Google Scholar]
- An in-house multiplex PCR test for the detection of Mycobacterium tuberculosis, its validation & comparison with a single target TB-PCR kit. Indian J Med Res. 2012;135:788-94.
- [Google Scholar]
- Diagnosis of tuberculosis by PCR-based amplification of mpt64 gene from peripheral blood. Int J Biomed Lab Sci. 2013;2:25-30.
- [Google Scholar]
- High degree of multi-drug resistance and hetero-resistance in pulmonary TB patients from Punjab state of India. Tuberculosis (Edinb). 2014;94:73-80.
- [Google Scholar]
- Multiplex PCR assay for simultaneous detection and differentiation of Mycobacterium tuberculosis, Mycobacterium avium complexes and other mycobacterial species directly from clinical specimens. J Appl Microbiol. 2009;107:425-35.
- [Google Scholar]
- Non-tuberculous mycobacteria in TB-endemic countries: Are we neglecting the danger? PLoS Negl Trop Dis. 2010;4:e615.
- [Google Scholar]
- Characteristics and early outcomes of patients with Xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin Infect Dis. 2012;54:1071-9.
- [Google Scholar]
- Diagnostic accuracy of Xpert MTB/RIF for extrapulmonary tuberculosis specimens: Establishing a laboratory testing algorithm for South Africa. J Clin Microbiol. 2014;52:1818-23.
- [Google Scholar]
- Comparison of Xpert MTB/RIF with line probe assay for detection of rifampin-monoresistant Mycobacterium tuberculosis. J Clin Microbiol. 2014;52:1846-52.
- [Google Scholar]
- Performance of Xpert MTB/RIF assay in diagnosis of pleural tuberculosis by use of pleural fluid samples. J Clin Microbiol. 2015;53:3636-8.
- [Google Scholar]
- Deciphering the sequential events during in vivo acquisition of drug resistance in Mycobacterium tuberculosis. Int J Mycobacteriol. 2014;3:36-40.
- [Google Scholar]
- Usefulness of multiplex PCR in the diagnosis of genital tuberculosis in females with infertility. Eur J Clin Microbiol Infect Dis. 2013;32:399-405.
- [Google Scholar]
- Extrapulmonary tuberculosis: New diagnostics and new policies. Indian J Chest Dis Allied Sci. 2014;56:71-3.
- [Google Scholar]
- Diagnostic significance of ascites adenosine deaminase levels in suspected tuberculous peritonitis in adults. J Microbiol Infect Dis. 2013;3:104-8.
- [Google Scholar]
- Diagnostic challenges of tuberculosis peritonitis in patients with and without end-stage renal failure. Clin Infect Dis. 2007;45:e141-6.
- [Google Scholar]
- Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non-respiratory samples: A systematic review. BMC Infect Dis. 2014;14:709.
- [Google Scholar]
- Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: A systematic review and meta-analysis. Eur Respir J. 2014;44:435-46.
- [Google Scholar]
- Xpert MTB/RIF Implementation Manual: Technical and Operational “How-To”. Geneva: World Health Organization; 2014.
- Xpert MTB/RIF: A new pillar in diagnosis of extrapulmonary tuberculosis? J Clin Microbiol. 2011;49:2540-5.
- [Google Scholar]
- Evaluation of the GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. J Clin Microbiol. 2011;49:4138-41.
- [Google Scholar]
- Evaluation of Xpert MTB/RIF assay for rapid molecular diagnosis of tuberculosis in a two-year period in Croatia. Int J Mycobacteriol. 2013;2:179-82.
- [Google Scholar]





