Translate this page into:
Serum Levels of Lipids, Calcium and Magnesium in Women with Hypothyroidism and Cardiovascular Diseases
Address for correspondence: Mr. Hussein Kadhem Al-Hakeim, E-mail: headm2000@yahoo.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Lipid abnormalities in hypothyroidism contribute to the disproportionate increase in cardiovascular risk. A possible relationship between serum level of magnesium (Mg) and calcium (Ca) and cardiovascular disease was recorded. In this work, the possible correlation between lipid profile components and serum cations Ca and Mg was investigated. Matched healthy women were evaluated in a cross-sectional study. All parameters were measured spectrophotometrically. The results showed a significant decrease (P < 0.05) in high-density lipoprotein-cholesterol (HDL-C), total and ionized Mg in hypothyroid patients in comparing with control group. There was a significant increase (P <0.05) in serum total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C) and (LDL-C)/(HDL-C) ratio in hypothyroid patients as compared with control group. However, no correlation was found between the cation levels and lipid profile of the studied groups. It can be concluded that patients with hypothyroidism exhibited elevated atherogenic parameters (TC and LDL-C) and high risk of cardiovascular diseases.
Keywords
Calcium
cholesterol
hypothyroidism
lipid
magnesium
INTRODUCTION
Decreased thyroid hormone synthesis and low levels of circulating thyroid hormones result in biochemical and/or clinical hypothyroidism. Hypothyroidism is a common disorder. The clinical hypothyroidism occurs in 1.5-2% of women and 0.2% of men.[1] In hypothyroidism patients, there is a negative effect on the lipoprotein profile [low high-density lipoprotein-cholesterol (HDL-C) and high total cholesterol (TC) and low-density lipoprotein-cholesterol (LDL-C)] and may translate into a sizable cardiovascular risk if left untreated.[2] Lipid abnormalities are reported to be more common in patients with overt hypothyroidism and are thought to contribute to the disproportionate increase in cardiovascular risk.[3] Hence, hypothyroid patients are at high risk of cardiovascular diseases.[4] Levothyroxine replacement therapy significantly improved the lipid profile in hypothyroid patients[5] indicating the possible relation between thyroid hormones and lipid levels in the blood of hypothyroid patients. In observational studies, significant inverse associations of blood pressure with dietary intake of magnesium (Mg), potassium, Calcium (Ca), fiber and protein have also been reported.[6] Several experimental and clinical studies suggest that Ca depletion elevates blood pressure.[7] Although the changes in various analytes, including Mg and Ca, may be slight in thyroid diseases in some instances and may not be an acute problem for the patient, it is possible that these disturbances will be important for a patient in the long term.[8] A possible relationship between increased serum level of different substances including Ca and cardiovascular disease was recorded.[9] Magnesium ameliorates atherosclerosis and hypertension promotes coronary vasodilatation and unloading of the heart causing increase in its efficiency.[10] It is becoming clear that a large body of epidemiologic data supports the idea that lower than normal dietary intake of Mg can be a strong risk factor for hypertension and different heart diseases. Low-serum Mg appears often to be associated with arrhythmias, coronary vasospasm and high blood pressure.[10] From the above introduction, lipid profile and serum Ca and Mg are considered as risk factors for cardiovascular diseases. The aim of the present work is to determine whether there is any correlation between lipid profile components and serum cations Ca and Mg in hypothyroid women.
MATERIALS AND METHODS
Sixty-five women with hypothyroidism (mean ± standard deviation age = 23.3 ± 6.9 years, median = 24). Patients with hypothyroidism were recruited from a private clinical laboratory in Najaf, Iraq. Hypothyroidism was diagnosed according to the results of thyroid hormones [low T3 and T4, and high thyroid stimulating hormones (TSH)]. None of the patients had a history of coronary heart disease, acute illness, pregnancy or disorders that affect lipid metabolism (e.g. diabetes mellitus, renal failure, nephrotic syndrome or pancreatitis). None of the patients was on a thyroid hormone therapy or lipid-lowering agent at study entry. Control subjects were 34 age- and sex-matched healthy control women median age = 26 year. Blood samples were drawn randomly (not fasting).
Total T3 (normal range 0.6-1.7 ng/mL) and total T4 (normal range 4.8-12 ng/dL) were measured using ELISA kits supplied by BioCheck® -USA, while TSH ELISA kit (normal range 0.4-7 µIU/mL) supplied by MonoBind® -USA. All patients had the following hormone profile: T3 < 0.6 ng/ ml, T4 < 4.8 ng/dL and TSH > 7 µIU/ mL. While the hormone results of the control group were within the normal ranges of the supplied hormone kits.
Serum (TC) and triglycerides were determined by enzymatic colorimetric assay (Spinreact® -Spain). The cholesterol in the sample oxidized by the action of cholesterol oxidase enzyme into 4-Cholestenona and hydrogen peroxide which in turn reacts with phenol and 4-aminophenazone in the presence of peroxidase enzyme to produce red color. The intensity of the color formed is proportional to the cholesterol concentration in the sample. Triglycerides in serum incubated with lipoproteinlipase, liberate glycerol and free fatty acids. Glycerol is converted to glycerol-3-phosphate by glycerol kinase action. Glycerol-3-phosphate is then converted by glycerol phosphate dehydrogenase to dihydroxyacetone phosphate and hydrogen peroxide that produce red color as in cholesterol estimation assay.
HDL-C was determined after precipitation of other lipoproteins by sodium phosphotungstate with magnesium chloride reagent (Spinreact® -Spain). After removed by centrifugation the clear supernatant containing HDL-C is used for the determination of HDL-C by same method used for TC estimation. LDL-cholesterol was calculated using Friedewald's formula (LDL-C = TC minus HDL-C minus triglycerides/2.19). The measurement of Ca in the serum is based on formation of color complex between Ca and o-cresolphthalein in alkaline medium using reagents supplied by Spinreact® -Spain. The intensity of the color formed is proportional to the calcium concentration in the sample.
Serum Mg was measured spectrophotometrically using ready for use kit supplied by Human® Company-Germany. The principle of the method depends on the formation of colored complex between Mg ions with xylidyl blue in an alkaline medium. Glycoletherdiamine-N, N, Ñ, Ñ-tetra-acetic acid (GEDTA) is used as masking agent for Ca ions. The procedure includes the incubation of only 10µL of serum with one milliliter of the reagent for ten minutes at room temperature and the color intensity reflects the magnesium concentration in the serum. Serum ionized Ca level was estimated using the following equation:[11]
Ionized Ca= (6.25 × TC 2 (total protein) × 3/8)/ (total protein) + 6.5)
While serum Mg levels were calculated according to the following formula:[12]
Ionized Mg in mmol/L = (0.66 × (total Mg in mmol/L)) + 0.039
Statistical analysis was carried out using pooled t-test using EXCEL program (Microsoft Inc. USA). The difference is significant when P < 0.05.
RESULTS
There was a significant increase (P <0.05) in serum cholesterol, LDL-C and LDL-C/HDL-C ratio in hypothyroid patients as compared with control. A significant decrease (P < 0.05) was also observed in the HDL-C levels in hypothyroid patients in comparison to control group [Figure 1]. A significant decrease in ionized Ca, total and ionized Mg [Table 1] in hypothyroid patients in comparing with control group. There is no significant differences noticed between hypothyroid and healthy groups in serum level of triglyceride, VLDL, total Ca, total and ionized Ca/Mg ratios.
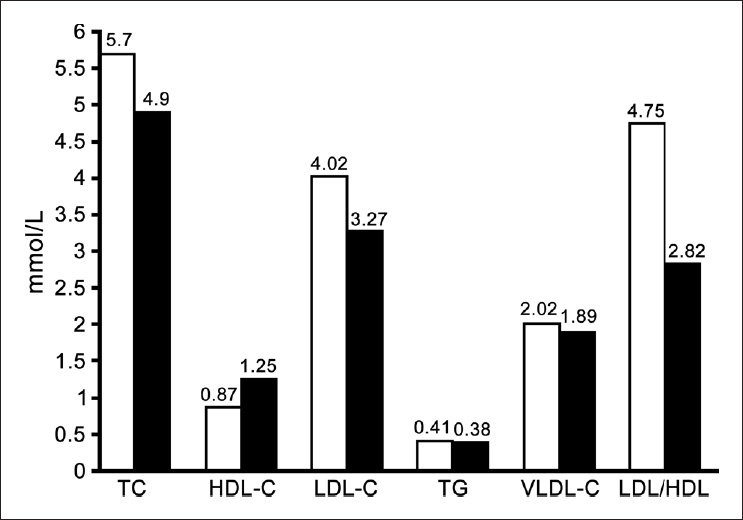
- Serum lipid parameters concentration in mmol/L of hypothyroid group (White bars) and normal control group (Black bars)
| Biochemical parameters | Patients | Control | P value |
|---|---|---|---|
| Total Ca (mmol/L) | 2.01 ± 0.35 | 2.19 ± 0.24 | 0.081 |
| Ionized Ca (mmol/L) | 0.88 ± 0.27 | 0.97 ± 0.24 | 0.138 |
| Total Mg (mmol/L) | 0.89 ± 0.19 | 1.01 ± 0.22 | 0.020* |
| Ionized Mg (mmol/L) | 0.57 ± 0.12 | 0.65 ± 0.13 | 0.019* |
| Total Ca/Mg | 2.39 ± 0.71 | 2.24 ± 0.48 | 0.173 |
| Ionized Ca/Mg | 1.54 ± 0.36 | 1.59 ± 0.37 | 0.422 |
*The difference is significant (P < 0.05)
The correlation coefficient values (not listed) showed no correlation between the serum cations, (Ca and Mg) concentration and every parameter of lipid profile (cholesterol, TG, VLDL, LDL-C, HDL-C, LDL-C/HDL-C ratio) in the studied groups.
DISCUSSION
The changes in lipid profile components noticed in the present study are common abnormalities of lipoprotein metabolism associated with hypothyroidism. The elevation in TC and LDL-C in hypothyroidism [Figure 1] is accounted for by the effect of thyroid hormone on lipoprotein-lipase activity[13] and the expression of the LDL receptor,[14] and these changes probably play an important role in atherogenesis in untreated hypothyroidism. Triglycerides and LDL-C levels were significantly high in hypothyroid patients when compared with the controls.[15] In hypothyroidism, the coronary lipid risk factors seem to be associated with lipid peroxidation indicating the presence of oxidative stress in hypothyroid patients and its association with atherogenic dyslipidemia.[16] Thyroid hormones have profound effects on the cardiovascular system.[17] By affecting the metabolism of lipids, hypothyroidism accelerates the process of atherogenesis and elevates cardiovascular risk. Although LDL is widely accepted as the major atherogenic lipoprotein, TG-rich lipoproteins such as chylomicron remnants, whose clearance is reduced in hypothyroidism[18] and very LDL remnants still play an important role in atherogenesis. These remnants are taken up by macrophages in the arterial walls to produce foam cells and thus may be a risk factor for atherosclerosis.[19]
There is a positive association between serum TSH levels and TC and LDL-C levels. As TSH increases, consequently TC and LDL-C rise as noticed in the present work. Thyroid hormones increase adipose tissue lipolysis as measured by high free fatty acids or glycerol release.[20] The total content of saturated fatty acids (FA) in serum was found to be high and the total content of unsaturated FA was low in hypothyroid patients.[21] From the above facts, the high body weight and high atherogenic lipids in hypothyroidism can be explained indirectly as a subsequence of the reduction of thyroid hormones.
Changes in LDL-C are mainly attributable to altered clearance of LDL-C from plasma by changes in the number of LDL receptors on liver cell surfaces.[22] Because the promoter of the LDL receptor gene contains a thyroid hormone responsive element, T3 could modulate gene expression of the LDL receptor.[23] HDL-C metabolism is complex and changes in plasma levels are due in part to remodeling of HDL-C particles by hepatic lipase and cholesterol ester transfer protein.[24] Activity of both enzymes is low in hypothyroidism and high in hyperthyroidism, correlating with plasma HDL-C.
The hypercholesterolemia is an important factor of atherosclerosis. High-density lipoprotein are particles with numerous atheroprotective functions, including facilitation of reverse cholesterol transport, improvement of endothelial function, protection of LDL from oxidation, limitation of hemostasis and retardation of inflammatory activity related to the vascular wall.[25] The reduction in HDL-C causes an increase in the LDL-C/HDL-C ratio, atherogenic index, which is an important prognostic marker for cardiovascular disease.
The significant changes in ionized Ca, but not total Ca [Table 1], means that the physiologically active form of Ca is affected, while the overall concentration of Ca is still significantly unchanged. Although there is no correlation between serum Ca and the components of lipid profile, the interest in serum Ca in hypothyroidism is still an attractive issue through the effect of Ca on the hypertension and heart muscle. Segal[26] study has provided a conclusive evidence for two central issues: That Ca is the first messenger for the plasma membrane-mediated action of thyroid hormone to increase cellular sugar uptake, and that thyroid hormone produces an acute increase in Ca uptake by the heart, an effect that is demonstrable at physiological concentrations and, therefore, points to a physiological relevance for this action.[26] Ca has an effect on the hypertension through the fact that, Ca load leads to the increment in Na excretion[27] and a reduced sodium intake reduces Ca excretion[28] and vice versa and hence decrease in hypertension.
Evaluation of serum Mg concentration has become more important in recent years in human.[29] The ionized form of serum Mg is believed to be a physiologically active component and hence can be measured or calculated.[12] Serum Mg in some papers showed a negative correlation with the level of thyroid hormones. Total serum Mg[30] and ionized and bound plasma Mg[31] concentrations were low in hyperthyroid patients. Thyroid hormone affects the glomerular filtration rate, blood flow and tubular sodium transport and has a direct effect on tubular Ca and Mg resorption.[32] A complete explanation for changing in Mg (including high urinary excretion) in hyperthyroid patients has not been offered. Significant low-ionized serum Mg has been documented in hyperthyroid patients.[31] However, the correlation between serum Mg and hypothyroidism is not clear and, in the present work, is not changed significantly.
CONCLUSION
There is no correlation between the concentration of serum cations, Ca and Mg, with every parameter of lipid profile in the studied groups. The fact that patients with hypothyroidism exhibited elevated atherogenic parameters and at high risk of cardiovascular diseases is further confirmed. Calcium has an effect on the hypothyroid patients; there is even no correlation with the lipid profile parameters. Therefore, treatment and follow-up of hypothyroidism should include the monitoring of serum Mg and Ca in order to decrease the possible effect of changing in the level of these cations on the risk of cardiovascular diseases in the hypothyroid patients.
Source of Support:
Nil
Conflict of Interest:
None declared.
REFERENCES
- American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthroidism and hypothryroidism. Endocr Pract. 2002;8:458-69.
- [Google Scholar]
- Increased atherogenic low-density lipoprotein cholesterol in untreated subclinical hypothyroidism. Endocr Pract. 2008;14:570-5.
- [CrossRef] [PubMed] [Google Scholar]
- Hyper-homocystinemia and hypercholesterolemia associated with hypothyroidism in the third US National Health and Nutrition Examination Study. Atherosclerosis. 2001;155:195-200.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma homocysteine concentrations and serum lipid profile as atherosclerotic risk factors in subclinical hypothyroidism. Ann Saudi Med. 2008;28:96-101.
- [CrossRef] [PubMed] [Google Scholar]
- Lipid profile in different degrees of hypothyroidism and effects of levothyroxine replacement in mild thyroid failure. Transl Res. 2008;151:224-31.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma status of selected minerals in hypertensive men with and without insulin resistance. J Trace Elem Med Biol. 2000;14:228-31.
- [CrossRef] [PubMed] [Google Scholar]
- Calcium metabolism and hypertension. Kidney Int. 1989;35:717-36.
- [CrossRef] [PubMed] [Google Scholar]
- Disturbances of calcium and magnesium metabolism occur in most hyperthyroid patients. Clin Biochem. 1989;22:373-6.
- [CrossRef] [PubMed] [Google Scholar]
- Serum 25-hydroxyvitamin D3 levels are elevated in South Indian patients with ischaemic heart disease. Eur J Epidemiol. 2001;17:567-71.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of Mg2+ on cardiac performance, intracellular free Mg2+ and pH in perfused hearts as assessed with 31P-NMR spectroscopy. Magnes Trace Elem. 1992;10:99-16.
- [Google Scholar]
- The simulaneous measurement of ionized and total calcium and ionized and total magnesium in intensive care unit patients. J Critical Care. 2002;17:203-5.
- [CrossRef] [PubMed] [Google Scholar]
- Serum lipoprotein and apolipoprotein concentrations and tissue lipoprotein-lipase activity in overt and subclinical hypothyroidism: The effect of substitution therapy. Eur J Clin Invest. 1981;11:3-10.
- [CrossRef] [PubMed] [Google Scholar]
- Alterations in thyroid status modulate apolipoprotein, hepatic triglyceride lipase, and low density lipoprotein receptor in rats. Endocrinology. 1990;127:1144-52.
- [CrossRef] [PubMed] [Google Scholar]
- Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. Clin Endocrinol (Oxf). 2008;34:45-52.
- [Google Scholar]
- Association between oxidative stress and coronary lipid risk factors in hypothyroid women is independent of body mass index. 14: Metabolism. 2007;56:1350-5.
- [CrossRef] [PubMed] [Google Scholar]
- Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501-9.
- [CrossRef] [PubMed] [Google Scholar]
- Serum concentrations of remnant-like particles in hypothyroid patients before and after thyroxine replacement. Clin Endocrinol (Oxf). 2003;58:621-6.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma lipoproteins and progression of coronary artery disease evaluated by angiography and clinical events. Circulation. 1993;88:2762-70.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated regional lipolysis in hyperthyroidism. J Clin Endocr Metab. 2002;87:4747-53.
- [CrossRef] [PubMed] [Google Scholar]
- Fatty acid composition of the blood serum and erythrocyte membrane lipids in patients with hypothyroidism and left ventricular diastolic dysfunction. Klin Med (Mosk). 2008;86:40-3.
- [Google Scholar]
- Structure and regulation of the LDL receptor and its gene. Br Med Bull. 1990;46:891-16.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of triiodothyronine and amiodarone on the promoter of the human LDL receptor gene. Biochem Biophys Res Commun. 1998;249:517-21.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma cholesterol transfer protein. J Lipid Res. 1993;34:1255-74.
- [CrossRef] [PubMed] [Google Scholar]
- Low high-density lipoprotein cholesterol as a risk factor in coronary heart disease: A working group report. Circulation. 2001;103:2213-8.
- [CrossRef] [PubMed] [Google Scholar]
- Calcium is the first messenger for the action of thyroid hormone at the level of the plasma membrane: First evidence for an acute effect of thyroid hormone on calcium uptake in the heart. Endocrinology. 1990;126:2693-702.
- [CrossRef] [PubMed] [Google Scholar]
- Potassium depletion and salt sensitivity in essential hypertension. J Clin Endocr Metab. 2001;86:2857-62.
- [CrossRef] [PubMed] [Google Scholar]
- The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr. 2003;133:3130-6.
- [CrossRef] [PubMed] [Google Scholar]
- The therapeutic use of magnesium in anesthesiology, intensive care and emergency medicine: A review. Can J Anaesth. 2003;50:732-46.
- [CrossRef] [PubMed] [Google Scholar]
- Magnesium metabolism in hyperthyroidism. Endocr J. 1996;43:397-402.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of stress and triiodothyronine on plasma magnesium fractions. Life Sci. 1994;55:327-32.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of thyroid status on renal calcium and magnesium handling. Can J Comp Med. 1984;48:51-7.
- [Google Scholar]





