Translate this page into:
Unrevealing the role of miRNA in successful TNBC treatment: A pilot study to explore the chemotherapy drugs for timely treatment of TNBC
*Corresponding author: Rashmi Chowdhary, PhD, Department of Biochemistry, All India Institute of Medical Sciences (AIIMS), Bhopal 462026, Madhya Pradesh, India rashmi.biochemistry@aiimsbhopal.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Sarkar P, Chowdhary R, Yadav AK, Arya N, Pandya B, Kumar V, et al. Unrevealing the role of miRNA in successful TNBC treatment: A pilot study to explore the chemotherapy drugs for timely treatment of TNBC. J Lab Physicians. 2024;16:63-73. doi: 10.1055/s-0043-1774405
Abstract
Objectives:
Worldwide, breast cancer is the most prevalent and common type of cancer. Physical examination and mammography with a range of sensitivities are currently used as screening methods. Triple-negative breast cancer (TNBC) lacks estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER-2) gene expression. MicroRNAs (miRNA) as potential prognostic and diagnostic biomarkers, miRNA 125, 200c, 221, 21, and 34a were selected for study.
Materials and Methods:
Here, 25 consenting TNBC patients with negative ER/PR/HER-2 status and compatible history were accrued from the Department of Oncosurgery, All India Institutes of Medical Sciences (AIIMS) Bhopal. Serum from participants and 25 controls was collected for quantitative estimation of miRNA by quantitative real-time polymerase chain reaction. After being treated with epirubicin, capecitabine, and paclitaxel, the MDA-MB-231 cell line’s expression of these miRNA subtypes was also examined.
Statistical Analysis:
All statistical analyses, pie charts, dot plots, and box-whisker plots were performed using EZR (Easy R), R Commander version 2.7-1. Bar graphs were created using Microsoft Excel 2019 software. Heat map graphics were produced using Graph Prism Version 9.
Results:
miRNA125 (p< 0.0001) and miRNA21 (p< 0.05) were found to be statistically significant. miR125 (DCt [cycle threshold] 2.77) was seen to be upregulated and miR21 (DCt -1.61) was seen to be downregulated in TNBC patients. Epirubicin treatment caused miR125 to be downregulated, but capecitabine treatment caused miR125 to be upregulated. Paclitaxel was seen to downregulate miR21. All three chemotherapeutic agents were seen to downregulate miR34a.
Conclusion:
miRNAs can be developed into a reliable biomarker and prognostic tool with more research. They can also help develop and improve pharmaco-therapeutic strategies.
Keywords
breast cancer
biomarker
tnbc
micrornas
diagnosis
chemotherapy
INTRODUCTION
Breast cancer remains leading form of cancer worldwide affecting large female population. As per clinical data, nearly 2.3 million breast cancer cases were reported/diagnosed in the year 2020 that leads to 0.7 million deaths. GLOBOCAN, 2020, estimated an alarming number of cases where 7.8 million females alive by the end of year undergone for clinical diagnosis in the last 5 years.[1] Triple-negative breast cancer (TNBC) continues to be the leading cause of cancer-related death in Indian women. In women with fatty breast parenchyma and thick breasts, respectively, conventional mammography screening sensitivity ranges from 98 to 36%.[2] Therefore, for the early diagnosis of breast cancer, the development of minimally invasive diagnostic tools is necessary. At least four clinically significant molecular subtypes of breast cancer are now recognized, including TNBC, human epidermal growth factor receptor 2 (HER-2)-enriched, luminal A, and luminal B.[3] TNBC is a heterogeneous subset of these tumors that is positive for basal cytokeratin ⅚ or estimated glomerular filtration rate but does not express estrogen receptors, progesterone receptors, or HER-2 gene amplification.[4] They are also very aggressive and typically affect young patients.[5]
Neoadjuvant chemotherapy may, in some cases, increase a patient’s eligibility for breast-conservation surgery with a decreased risk of local recurrence, but it may also be associated with unfavorable patient outcomes, especially in those who do not have a pathological complete response.[6,7] As a result, while a patient is receiving neoadjuvant chemotherapy, early identification of individuals who are not responding well to treatment enables the treatment to be changed effectively to address avoidable adverse effects of systemic therapy. Therefore, it is necessary to create minimally invasive markers with high specificity and sensitivity for tracking chemotherapy response as well as for spotting cancers that become resistant to systemic therapy.[8] MicroRNAs (miRNAs) have been investigated as potential predictive and diagnostic biomarkers in this regard in the past.[9,10] miRNAs are found in the extracellular space and as circulating molecules in bodily fluids such blood, serum, plasma, urine, tears, saliva, seminal fluid, cerebrospinal fluid, and extracellular fluid, among others.[11] miRNA dysregulation has a negative impact on the prognosis overall because it contributes to the emergence of treatment resistance and all types of cancer.[12,13]
Because of this, evaluating miRNA can help with treatment responsiveness, extensive surveillance of high-risk patients, and identifying patients with metastases. Identifying circulating miRNAs as prognostic indicators in TNBC as a result of neo-adjuvant chemotherapy is the aim of this study. This will be accomplished by comparing the expression of different miRNAs in TNBC patients to that in healthy females. This study aims to understand how chemotherapy exposure affects the expression of different miRNAs in breast cancer cell lines both before and after treatment with particular anticancer drugs.[13,14] Despite the fact that numerous studies have demonstrated that miRNAs can be successfully used for diagnostic and prognostic purposes in TNBC, there have not been many studies in an Indian context that have concentrated on the specific miRNAs expressed in TNBC in the Indian population, which can then be studied for specificity as a noninvasive diagnostic marker. In this study, much emphasis was given on has-mir-125, has-mir-34a, has-mir200c, has-mir-221, and has-mir-21. Additionally, this study also aims to evaluate in vitro expression of miRNAs on breast cancer cell lines and their correlation among drug treatment. Overall, the goal of this study is to analyze the expression of miRNAs and their response toward drugs.
MATERIALS AND METHODS
Study Design
After scrutinizing 100 breast cancer patients, 25 samples (n = 25) of serum from patients with TNBC who had not yet received treatment and 25 serum samples from healthy, normal women were obtained from the All India Institute of Medical Sciences (AIIMS), Bhopal, outpatient department (OPD). The patients were chosen at random as they arrived at the hospital’s Onco-surgery/Radiotherapy OPD. Collected blood was centrifuged at 3,000 g for 15 minutes at 4°C after standing at room temperature for at least 30 minutes. Isolated serum was then aliquoted (500 µL) into 1.5 mL Eppendorf tubes and kept at 80°C until RNA extraction.
Cell Culture and Cell Growth Assay
The National Centre for Cell Science, Pune provided the human breast cancer cell lines MDA-MB-231, which were cultured in Eagle’s modified essential medium at 37°C with 5% CO2. In 96-well plates, MDA-MB-231 and MDAMB-231/epirubicin, capecitabine, and paclitaxel were plated. Cell viability was assessed at 0, 24, 48, and 72 hours using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reagent (Keygen Biotech, Nanjing, China). By measuring the absorbance at 490 nm on enzyme-linked immunosorbent assay reader, cell viability was determined (BioTek).
Colorimetric MTT (Tetrazolium) Assay
MTT was used to assess the impact of three anticancer drugs, namely epirubicin, capecitabine, and paclitaxel, on the TNBC cell line MDA-MB-231. In 90 L of culture media, 5 103 MDA-MB-231 cells were seeded onto 96-well microtiter plates, and the plates were incubated overnight. The three anticancer drugs were applied to the cells in varied quantities for 24 hours. Each well received 10% v/v of the MTT solution, which was produced at 5 mg/mL in sterile phosphate buffered saline, and was then incubated at 37°C for 4 hours. To fully solubilize the formazan crystals, dimethyl sulfoxide was added, and the micro-plate reader was used to measure absorbance at 570 nm (BioTek). The number of reagents required to block 50% of cellular proliferation following a 48-hour treatment period is known as the half maximal inhibitory concentration (IC50). Epirubicin’s IC50 was determined to be 74 µM, capecitabine’s IC50 to be 85 µM, and paclitaxel’s IC50 to be 24µM.
Reverse Transcriptase PCR
The total RNA from breast cancer cell lines was extracted using Trizol method. Using the miRNAs sequence (Assay Technology), distinct miRNA primers for each of the five miRNAs were designed and synthesized, and then each miRNA’s unique annealing temperature was determined (Table 1). Using an avian myeloblastosis virus reverse transcriptase kit that is commercially available from GCC Biotech, 500 ng of total RNA was reverse transcribed. The reaction mixtures were then incubated at 16°C for 30 minutes, 42°C for 30 minutes, 85°C for 5 minutes, and then held at 4°C. Before usage, the produced cDNA was kept at -20°C.
| miRNA | Primer sequence |
|---|---|
| miR 34a-5p | 5′,-TGCGCTGGCAGTGTCTTAGCTG-3′ |
| miR 221–5p | 5′,-GCACCTGGCATACAATGTAGA-3′ |
| miR 125–5p | 5′,-TCCCTGAGACCCTAACTTG-3′ |
| miR 200c-3p | 5′,-TAATACTGCCGGGTAATGATGGA-3′ |
| miR-21–5p | 5′,-GCCCGCTAGCTTATCAGAC-3′ |
| U6 | 5′,-GCTTCGGCAGCACATATACTAAAAT-3′ |
Quantitative Real-Time
PCR Using the Sybr green matrix kit (Bio-Rad) and 20 ng cDNA per 20 µL reaction, real-time polymerase chain reaction (PCR) was performed individually for sera and cell lines with their respective normal controls. The real-time (RTPCR) cycles included 40 cycles of 95°C for 1 minute. and 58°C for 1 minutes, followed by predenaturation at 1 cycle of 95°C for 3 minutes. Every action, including the no-template controls, was performed twice. Fixed threshold settings were used to obtain the resulting Cq values. To maintain the same efficiency across all miRNA analyses, we have always employed the same concentration of cDNA. Based on the threshold cycle (Ct) value, the twofold change in miRNA expression for each breast tumor sample in comparison to the normal control was computed using the following formula: relative quantification (RQ) = 2 −DDCT. miRNA expression was standardized to serum volume because U6 and 5S rRNA are destroyed in serum samples and there isn’t a consensus housekeeping miRNA for the RT-quantitative PCR investigation of serum miRNAs.
Statistical Analysis
All statistical analyses, pie charts, dot plots, and box-whisker plots were performed using EZR (Easy R), R Commander version 2.7–1. Bar graphs were created using Microsoft Excel 2019 software. Heat map graphics were produced using Graph Prism Version 9. Using the Kolmogorov– Smirnov test for normal distribution, the normality of a continuous variable was evaluated. Mann–Whitney U test without parameters. The statistics were provided together with the median and interquartile range using the U test to determine the statistical significance between the two groups (interquartile range [IQR]). Statistical significance was defined as a p-value less than 0.05 (p = 0.05). To assess the predictive potential, receiver operating characteristic curve analysis and area under the curve (AUC) calculation were used.
RESULTS
Patient Demographics
Here in this study, 50 people in all were enrolled in the trial, of which 25 (the test group) had TNBC and 25 (the control group) were otherwise healthy people. In contrast to the healthy control group, which had a mean age of 41.68 years (standard deviation [SD]: 14.25 years), the test group had a mean age of 50 years (SD: 11.72 years) (Supplementary Figure S1). Here, 13 patients (52%) and 12 patients (48%) from the test group of this study’s participants were from rural areas, whereas all 25 of the healthy controls were from metropolitan areas. Among the patients enrolled in the test group, 11 (44%) were premenopausal women and 14 (56%) were postmenopausal women (Supplementary Table S1). Supplementary Figure S2 summarizes IC 50 values of anticancer drugs used in this study: a. Epirubicin, b; paclitaxel and c; capecitabine. Seven (28%) of the 25 TNBC patients recruited in this study had a confirmed family history of breast cancer, compared with 18 (72%) of the patients who had no such history (Supplementary Table S2).
Risk Factors and Comorbidities
As the result showed in Supplementary Table S3, three (12%) of the 25 TNBC patients participating in the trial disclosed a history of cigarette use, but no one disclosed a history of alcohol use. Of the 24 TNBC patients, 24 (96%) disclosed a history of pregnancy. Further, 4 (16%), 13 (52%), and 8 (32%) of the test group members had body mass indices (BMIs) of 18.5 to 22.9, 23 to 24.9, and less than 25, respectively. Eight (32%) had diabetes mellitus, five (20%) had hypertension, and two (8%) had hypothyroidism. Nine patients (36%) and 16 (64%) of the patients, respectively, were found to have hemoglobin levels below 12 mg/dL. As per data summarized in the Supplementary Table S4, only 3 (12%) of the patients in the test group had lesions in their right breast, compared with 22 (88%) who had involvement in their left breast. Eleven (44%) had stage II, 3 (12%) had stage III, and 11 (44%) had stage IV, according to the American Joint Committee on Cancer. There were 25 TNBC patients (100%) who were all involved in the lymph nodes. Eight (32%) of the patients who were enrolled in the test group experienced metastases, while 17 (68%) did not.
Expression Profile of miRNAs
Here in this study selection of miRNAs for their expression profile analysis associated with the TNBC patients was performed. Previous studies have demonstrated role of mir-34a,[15] miR125,[16–18] miR-21,[19,20] miR221,[21] and mir-200[22–24] miRNAs in the development of TNBC. The miRNA mean cycle threshold (Ct) levels were calculated independently for patients and controls (Supplementary Table S5, S6). By deducting the D Ct values of the controls from the Ct values of the cases, the Ct value was computed. miR125 had a Ct of 2.77, miR34a a Ct of -1.31, miR200c a Ct of 0.71, miR21 a Ct of 1.61, and miR221 a Ct of -0.88 (Tables 2 and 3). The difference between cases and samples for miR125 (median: 36.62, IQR: 3.365) and miR21 (median: 36.285, IQR: 2.7225) was found to be statistically significant (p< 0.0001 and p< 0.005, respectively). For miR34a, miR200c, and miR221, the difference between cases and samples was found to be not statistically significant (Table 4).
| miRNA | Mean Ct (Control) | Mean Ct (Cases) | D Ct |
|---|---|---|---|
| miRNA 125 | 39.7024 | 36.9388 | 2.77 |
| miRNA 34a | 37.28 | 38.598 | —1.31 |
| miRNA 200c | 13.1792 | 13.8828 | —0.71 |
| miRNA 21 | 35.5008 | 37.1144 | —1.61 |
| miRNA 221 | 28.1516 | 29.0376 | —0.88 |
Ct, cycle threshold; miRNA, microRNA; PCR, polymerase chain reaction.
p-Value<0.05.
| miRNA | Median | Interquartile range (IQR) | p-Value |
|---|---|---|---|
| miRNA 125 | 38.62 | 3.365 | <0.0001mmmm |
| miRNA 34a | 39.315 | 2.945 | 0.764 |
| miRNA 200c | 30.06 | 1.49 | 0.159 |
| miRNA 21 | 36.285 | 2.7225 | <0.05m |
| miRNA 221 | 27.565 | 1.8375 | 0.0859 |
p-Value<0.05, miRNA: micro RNA, IQR: Interquartile range.
| U6 | mir200c | mir21 | mir221 | mir34a | mir125 | |
|---|---|---|---|---|---|---|
| Control | 18.53 | 28.3 | 31.6 | 22.7 | 11.5 | 28.42 |
| Epirubicin (Epi) | 24.16 | 27.9 | 31.8 | 23.21 | 38.5 | 36.09 |
| Capecitabine (Cap) | 23.23 | 28.15 | 32 | 29.9 | 35.8 | 28.91 |
| Paclitaxel (Pac) | 11.73 | 29.9 | 32.8 | 26.2 | 24.2 | 19.3 |
Abbreviations: Ct, cycle threshold; miRNA, microRNA.
Evaluation of the Diagnostic Potential of miR-125, miR-34a, miR-21, miR-200c, and miR221 in the Serum of TNBC Patients
MiR125 was seen to have an AUC of 0.698 (95% confidence interval [CI]: 0.548–0.847), miR34a had an AUC of 0.524 (95% CI: 0.362–0.686), miR21 was seen to have an AUC of 0.962 (95% CI: 0. 913–1.000), miR200c had an AUC of 0.616 (95% CI: 0.447–0.785), and miR221 had an AUC of 0.642 (95% CI: 0.486–0.799). Euclidean hierarchical complete linkage clustering based heat map showed in Figure 1A for the selected 5 circulating miRNA (miR21, miR 221, miR200c, miR125, and miR34a). Additionally, a comparison of expression profile of miR21, miR 221, miR200c, miR125 and miR34a associated with the breast cancer precisely TNBC shown in Figure 1B to E. After epirubicin treatment, a striking downregulation of miR125 was observed; however, capecitabine and paclitaxel showed no comparable downregulation changes. Capecitabine increased the expression of miRNA 125.
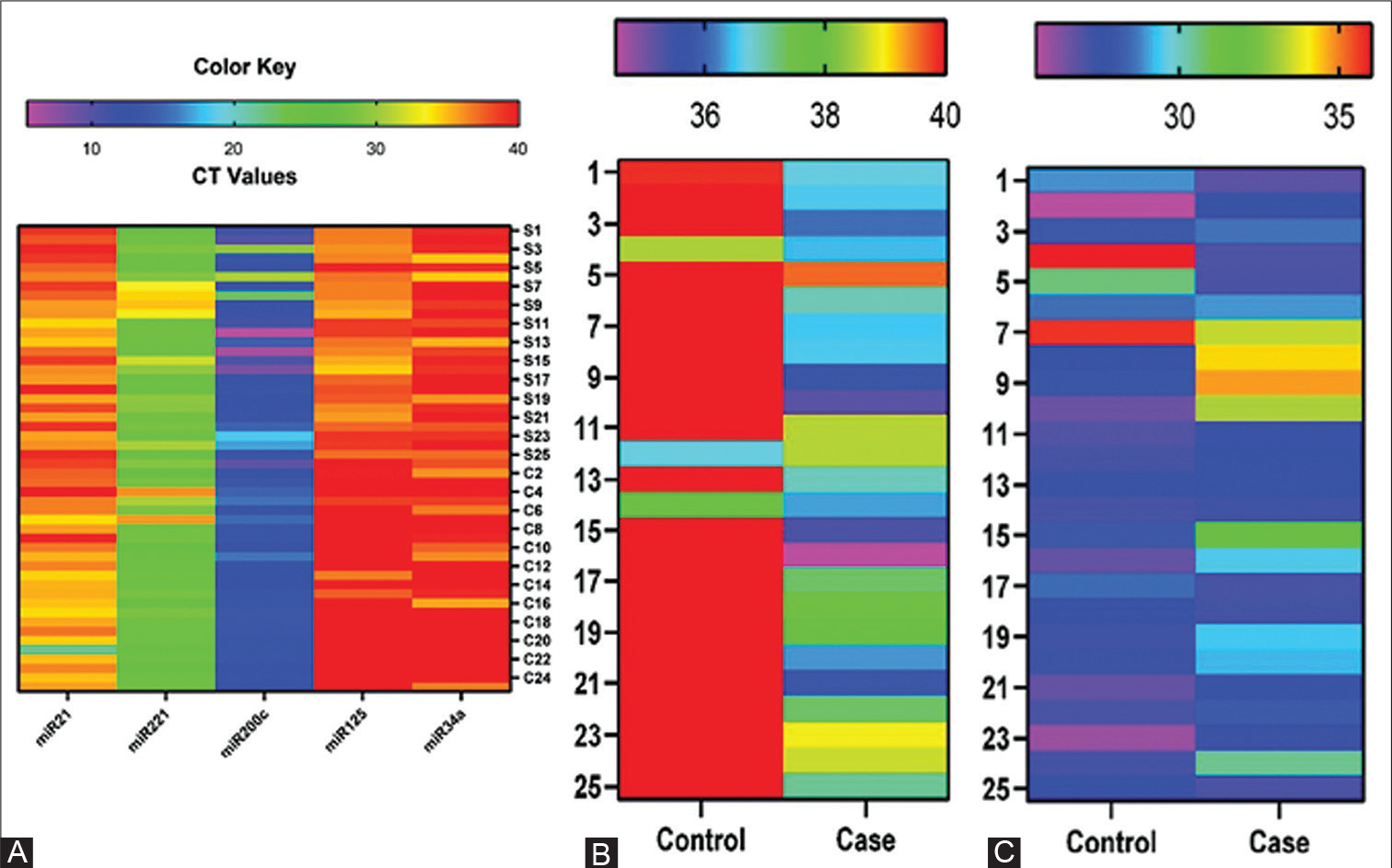
- (A) Heat map: Euclidean hierarchical complete linkage clustering based on the selected 5 circulating microRNA (miRNA), (B) Heat map of miRNA 125 of triple-negative breast cancer (TNBC) patient samples and normal healthy control serum samples. (C) Heat map of miRNA 221 of TNBC patient samples and normal healthy control serum samples. Ct, cycle threshold.
It was observed that paclitaxel downregulated miR200c, miR21, and miR221 (Figure 1A–E). It has been observed that miR34a expression is downregulated by all three chemotherapy drugs. Epirubicin was reported to induce the highest amount of cell death after anticancer chemotherapeutic therapy (Figure 3). Paclitaxel was shown to have the second-highest rate of cell death following anticancer chemotherapeutic therapy (Figure 4). Capecitabine caused quite minimal cell death after anticancer chemotherapeutic therapy (Figure 4 and Table 4). Additionally, Supplementary Table S7 summarizes miRNAs expression and twofold change of MDA-MB-231 after treatment with anticancer chemotherapeutic agents according to their respective IC50 dose for 24 hours (Supplementary Figure S2). A differential expression profile of miR21, miR221, miR200c, miR125 and miR34a was reported in MDA-MB-231 cell lines after the treatment of anticancer chemotherapeutic agents (Figure 2B).
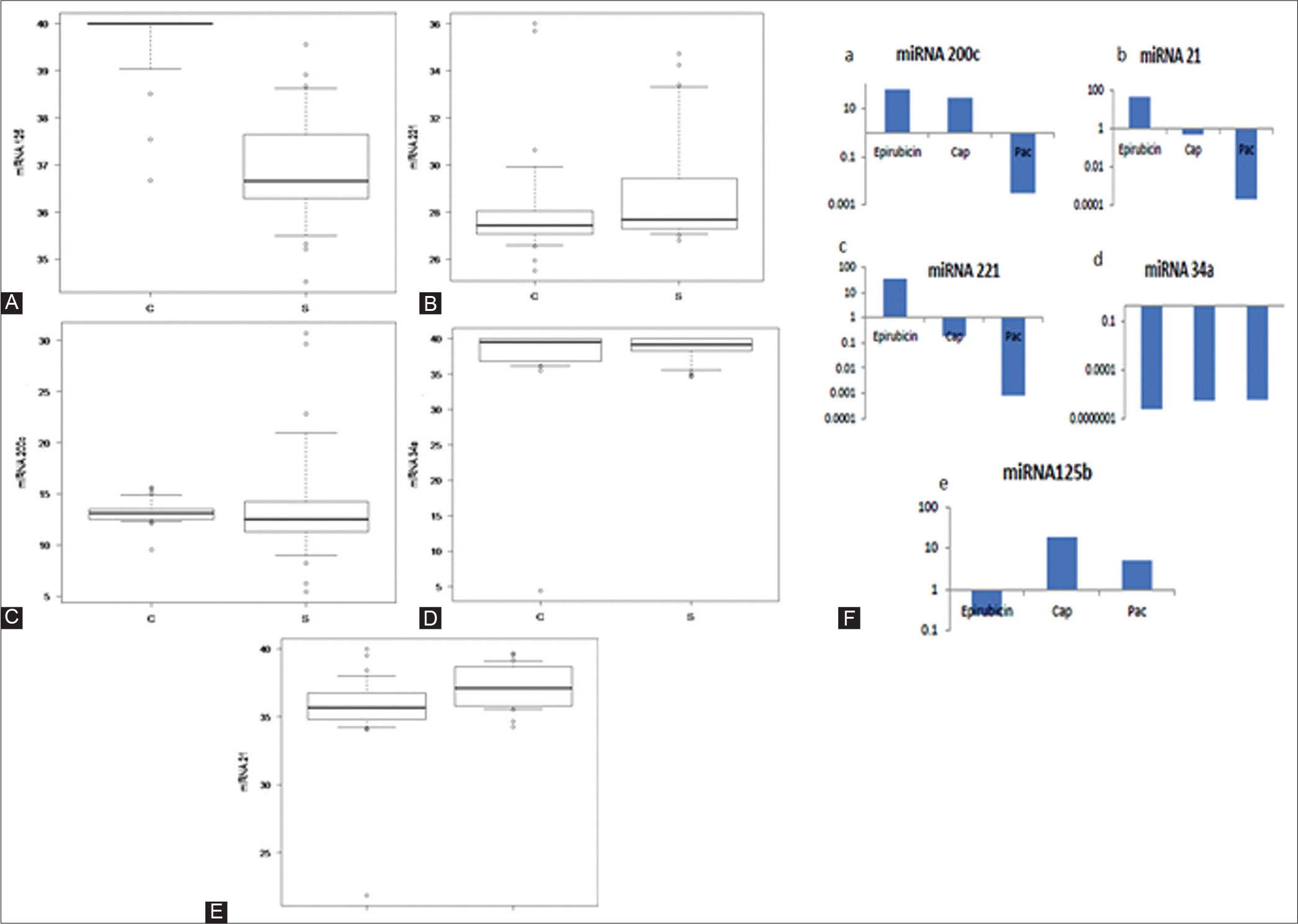
- Box plot: Triple-negative breast cancer patients versus healthy controls: microRNA (miRNA) expression in serum sample. Box-whisker plot showing Ct values and its corresponding p-value; (A) miRNA125, (B) miRNA225, (C) miRNA200c, (D) miRNA 34a, and (E) miRNA 21, (F) Representation of miRNA expression and twofold change of MDA-MB-231 after treatment with anticancer chemotherapeutic agents; miRNA200c, miRNA21, miRNA221, miRNA 34a, and miRNA125b.
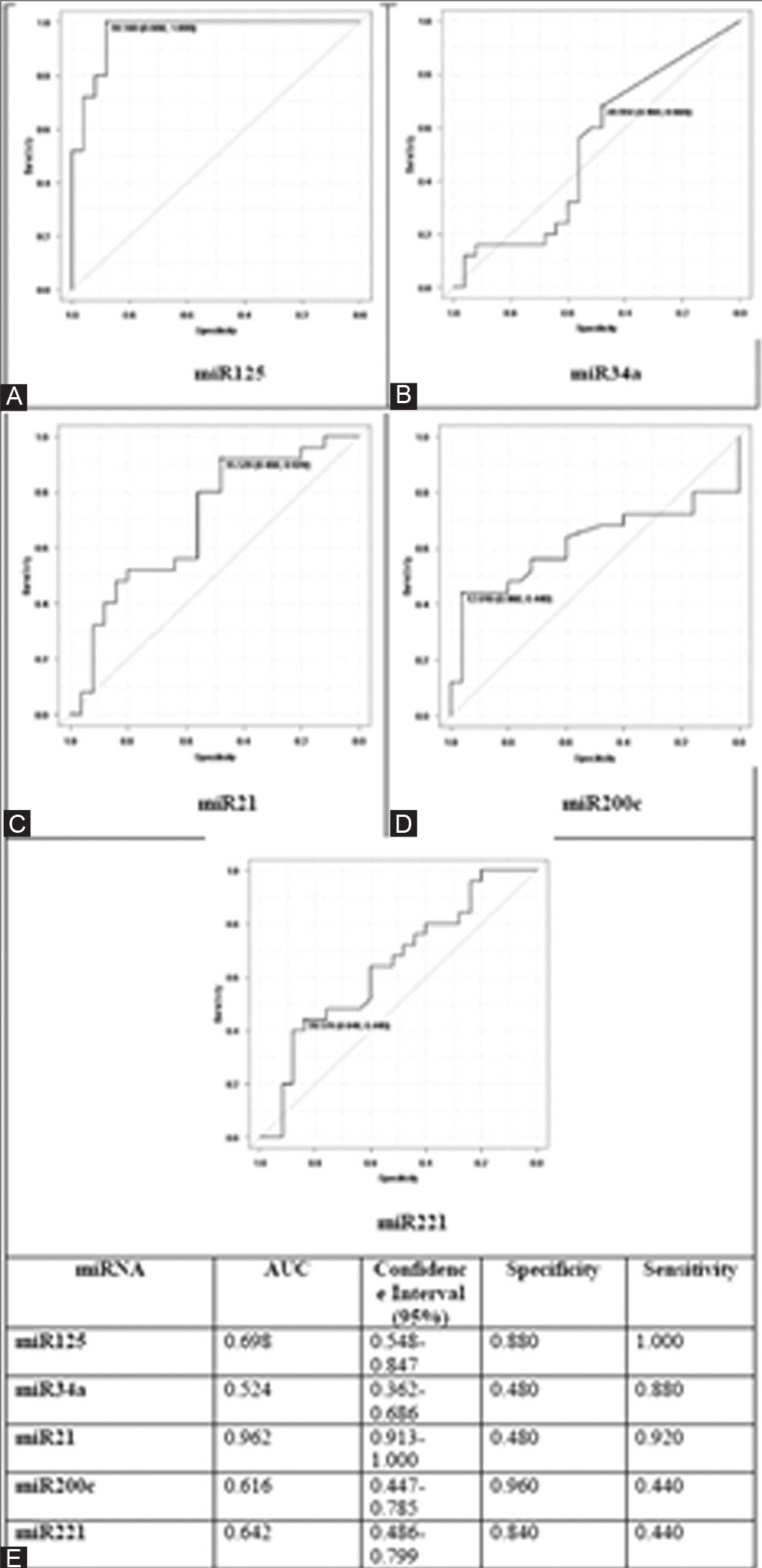
- (A) Evaluation of the diagnostic potential of microRNA-125 (miRNA-125), (B) miRNA-34a, (C) miRNA-21, (D) miRNA-200c, and (E) miRNA 221 in the serum of triple-negative breast cancer patients. (F) The therapeutic potential of miRNA-125, miRNA-34a, miRNA-21, miRNA-200c, and miRNA 221 was examined via determination of specificity and sensitivity of miRNAs in serum. AUC, area under the curve.
This study reported higher expression of miR21, miR221, miR200c, miR125, and miR34a with epirubicin treatment in cell line system MDA-MB-231 (Figure 4B).
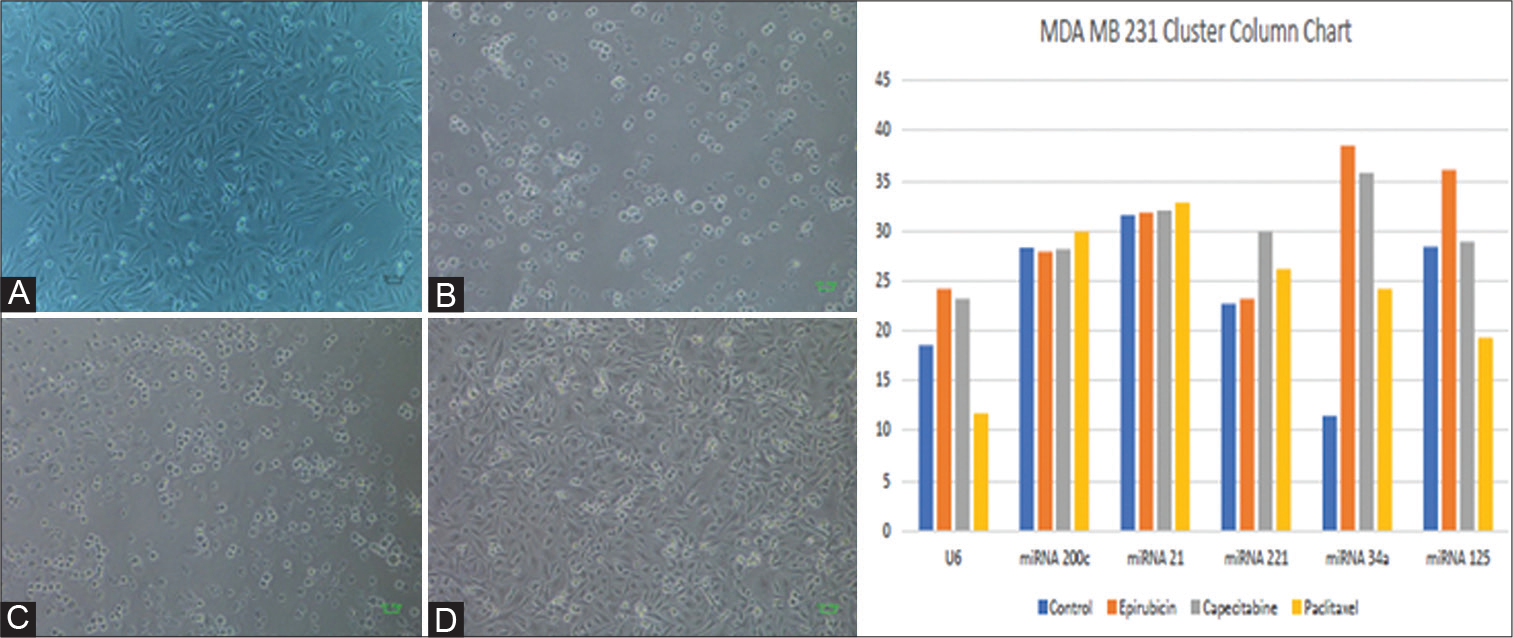
- (A) MDA-MB-231 cell phenotype under the exposure of chemotherapeutics; Microscopic image of MDA-MB-231 cell line pre-treatment with anticancer chemotherapeutic agent. (B) Microscopic image of cell death observed in MDA-MB-231 cell line following treatment with epirubicin. (C) Microscopic image of cell death observed in MDA-MB-231 cell line following treatment with paclitaxel. (D) Microscopic image of cell death observed in MDA-MB-231 cell line following treatment with capecitabine. (E) MDA-MB-231 cluster chart.
DISCUSSION
The purpose of this study was to assess the expression of miRNA in TNBC in patients receiving care at a tertiary care facility in central India. Even though there have been improvements in care strategies for breast cancer; it continues to be the biggest contributor to cancer-related deaths in Indian women.[14] Locally advanced breast cancer and TNBC are frequently treated with neoadjuvant chemotherapy, but the poor clinical outcome in TNBC has pushed for the development of predictive biomarkers for early detection of TNBC and for the assessment of these biomarkers’ responses to chemotherapeutic treatment in TNBC patients because there are so few established prognostic and predictive biomarkers.[25] Twenty-five TNBC patients who had serum samples taken before beginning neoadjuvant chemotherapy, radiation, and surgery made up the test group. Twenty-five healthy women who had no family history of uterine, ovarian, or breast cancer made up the control group. The TNBC patients in this study ranged in age from 26 to 75 years old, with a median age of 50.04 years (SD: 11.72 years). Other research done in India revealed that patients were diagnosed with TNBC on average at 48.41 and 48.8 years old, respectively. Despite the fact that TNBC affects women of various ages, there is no clear association between the prognosis and the age of diagnosis.[26] The most frequent cancer now diagnosed worldwide is breast cancer.1 In this study, 12 patients (48%) came from metropolitan areas, whereas 13 patients (52%) came from rural areas.[27]
Lack of knowledge of breast cancer in the rural population, which is reflected in the high mortality-to-incidence ratio, is another explanation for the discrepancy that skews the incidence of TNBC or breast cancer as a whole toward a more urban population.[28,29] Here, 11 (44%) of the TNBC patients who participated in our study were premenopausal, whereas 14 (56%) patients were postmenopausal. Family history plays a big role in predicting the likelihood of having TNBC.[30] Only 7 (28%) of the TNBC patients in our study provided a positive family history, while 18 (72%) of the patients provided a negative one. This is in contrast to other research’ findings, which indicated a high association between a family history of breast cancer and its progression, particularly TNBC.[31,32] It can also predispose to diabetes mellitus and cardiovascular complications. In our investigation, five (20%) of the subjects had known cases of hypertension, while eight (32%) patients were found to have a BMI below 25. Eight (32%) of the TNBC patients had a history of type II diabetes. Although type II diabetes and insulin resistance are both connected to an increase in the overall incidence of breast cancer, TNBC is less frequently associated with type II diabetes.[33,34] In this study, only three (12%) of the TNBC patients had involvement of the right breast, while 22 (88%) of the TNBC patients had lesion involving the left breast. This is consistent with Perkins et al 2004[35] and other studies[36,37] found that the left breast was more affected than the right breast. Additional investigation into the physiological and immunological pathways may assist to elucidate the issue, even though the precise source of the phenomenon is yet unknown.[38]
According to the clinical staging of the TNBC patients included in this study, 11 (44%) patients were in stage II, 3 (12%) were in stage III, and 11 (44%) were in stage IV. Similar patterns were observed in patients reporting with clinical stage II in studies by Nabi et al[34] and Cornier et al. 2008,[39] although in those studies the trend of patients diagnosed with stage III was noticeably higher, the trend of patients diagnosed with stage IV was noticeably lower. This discrepancy can be explained by the higher proportion of patients from rural areas, who often only seek care when very ill due to ignorance and financial constraints.[40] Patients with TNBC are more likely to have lymph node involvement, and the degree of lymph node involvement affects the course of treatment and the prognosis.41 In this investigation, lymph nodes were involved in all 25 (100%) individuals. Studies by Akhtar et al[27] and Nabi et al[34] also demonstrated tendencies of greater lymph node involvement in TNBC patients. TNBC has a higher propensity for distant metastasis because of its high level of malignancy and invasiveness. This increased propensity manifests itself in organs like the liver, lungs, bones, and portions of the central nervous system, which has a detrimental effect on the patient’s prognosis.[40] Eight (32%) of the total patients had distant metastases upon presentation, which is consistent with the proportion of patients with advanced clinical stages.
Circulating miRNAs are an additional, if not a substitute, diagnostic technique for detecting breast cancer early, which opens the door for a better prognosis.[41] This is due to their distinctive expression profiles and exceptional stability in plasma. A work by Guo et al has shown the prospect that certain circulating miRNAs can be employed as better diagnostic biomarkers for malignant lesions of the breast in comparison to traditionally used tumor markers like C15– 3 and CEA.[42] Twenty-five women with TNBC who were receiving OPD-based treatment and 25 healthy women were enrolled in this study.[43] Data on their ages, racial, and ethnic backgrounds, menopausal status, and family history of breast cancer, history of pregnancies, addictions, BMI, and comorbid conditions like hypertension, diabetes mellitus, and hypothyroidism were collected.[44] The expression of a total of five miRNAs—hsa-mir-125, hsa-mir-34a, hsa-mir-200c, hsa-mir-221, and hsa-mir-21—in the serum samples of each patient was assessed.[45]
miRNA 125 (p< 0.0001) and miRNA 21 (p< 0.05) were found to be statistically significant among the five miRNAs examined, with miR 125 (DCt 2.77) being observed to be upregulated and miR 21 (DCt -1.61) being seen to be downregulated in patients with TNBC, respectively.[46–48] The miRNA 125 family plays a variety of roles by targeting various areas critical in biological processes like the maintenance of normal homeostasis and proliferation of immune cells, altering cell differentiation, and apoptosis, which can contribute to tumorigenesis.[49] This is done through the process of post-transcriptional regulation of gene expression. The findings of Wang et al[50] revealed elevated miR125b levels in breast cancer patient blood samples that were inversely correlated with the grade of the tumor and the stage of lymph node metastasis, among other findings. Similar to this, Nie et al study revealed that TNBC patients had higher levels of miR125b in their blood.[51] In our study, we found that the miR125 was significantly elevated in TNBC patients, which is consistent with the results previously indicated.
It is widely known that miR21 plays a pro-oncogenic effect.[52] By inhibiting apoptosis, miR21 has been found to be upregulated in several solid tumors, including tumors of the gastrointestinal tract, prostatic, lung, and neuroendocrine systems.[53] In stark contrast to previously reported findings, miR21 was shown to be downregulated in our study (Ct -1.61, p 0.05). Further analysis is necessary because the precise mechanism responsible for the clarification of this finding remains unclear. We also looked at three other miRNAs: miR221 (may be tumor suppressor or oncogenic depending on the tumor),[54] miR200c (a circulating miRNA that prevents TNBC from spreading),[55] and miR34a (whose downregulation indicates a poor prognosis).[56] MiR221 was downregulated (DCt -0.88), as was miR200c (DCt -0.71) and miR34c (DCt -1.31), but these changes were not statistically significant (p = 0.086, p = 0.16, and p = 0.76, respectively). By calculating the AUC, ROC curve analysis can be used to evaluate a possible diagnostic tool’s discriminatory capacity between cases and control data.[56] With a specificity of 0.880 and a sensitivity of 1.000, miR125 in this investigation shown good predictive ability and had an AUC of 0.698 (95% CI: 0.548–0.847), which is consistent with the findings of Fang et al.[57] Since miR21, an oncogenic miRNA, was observed to be downregulated, a conclusion inferred from this data may be incorrect. MiR21 had an AUC of 0.962 (95% CI: 0.480–0.920), which also demonstrated a strong predictive power. Therefore, more analysis is necessary for the situation. In comparison to miR125, the AUC of miR34a, miR200c, and miR221 did not show a strong predictive power.
Monoclonal antibodies, one of the most effective therapeutic methods now available, are ineffective in TNBC tumors due to the lack of receptors, making the use of nonspecific cytotoxic medicines a necessity.[58] The development of chemotherapy resistance in TNBC is largely influenced by changes in the number and structure of chromosomes, as well as gene deletions or mutations like those in JAK2 and PTEN, immune evasion, and activation of pro-oncogenes such PIM1.[59] Pharmacotherapeutic result in TNBC patients can be improved by composite markers that can extrapolate chemotherapy efficacy while also predicting triple-negative malignant lesions.[60] The expression of the five miRNA subtypes was assessed on the MDA-MB-231 TNBC cell line after treatment with the three chosen anticancer agents, namely epirubicin, capecitabine, and paclitaxel; in study by Wang et al,[61] and miR21 was found to be a potential biomarker for the assessment of response to neoadjuvant chemoradiotherapy. After epirubicin treatment, a striking downregulation of miR125 was observed; however, capecitabine and paclitaxel showed no comparable downregulation changes. Epirubicin may have a better therapeutic effect when miR125 is downregulated because miR125 is oncogenic, but capecitabine may have a worse therapeutic outcome when miR125 is up-regulated.[62]
Similar to how paclitaxel was observed to downregulate miR200c, miR21, and miR221, whereas downregulation of miR200c, a tumor suppressor,[63] may negatively impact the treatment outcome and result in disease progression or chemoresistance. In this investigation, it was discovered that all three chemotherapy drugs reduced the activity of miR34a, a tumor suppressor[64] that may cause chemoresistance. As one of the “first of its kind” research to be undertaken in the institute and in India, there is still a wealth of knowledge to be learned about the patterns of miRNA expression in the Indian population.[65] Evaluation of the circulating miRNA status in TNBC patients in conjunction with assessment of the chemotherapeutic drug’s effect on the identified significant miRNAs may offer valuable insight in predicting the prognostic and pharmacotherapeutic outcome, which can be crucial in the formulation of a chemotherapeutic strategy tailored to each patient’s needs while minimizing the unfavorable therapeutic and prognostic outcomes. For this hypothesis to be established and validated to improve patient care, more research is necessary.
CONCLUSIONS
Due to their aggressive proliferation, high local infiltration, and potential for metastasis, TNBCs are a significant cause of neoplasm-related morbidity and mortality in women. Due to the dearth of receptors, targeted therapy is impractical, forcing nonspecific cytotoxic chemotherapy that raises morbidity. In this investigation, TNBC patients from central India had the expression of circulating miRNAs evaluated. We can confirm that miRNAs are trustworthy biomarkers as a screening and prognosis prediction tool based on blood tests that can support the already existing diagnostic modalities. These miRNAs can aid in the formulation and improvement in the pharmacotherapeutic strategy by forecasting pharmacotherapeutic response and the possible development of chemo-resistance. We next assessed whether these miRNAs were upor downregulated in response to three widely used chemotherapeutic anticancer drugs. This study provides some insight into the possibility of a relationship between the measurement of circulating miRNA expression and chemotherapeutic response, which may aid in the development of customized pharmacotherapies for the best possible patient care. However, more research is required to establish and validate circulating miRNAs as compound biomarkers.
Acknowledgment
Authors would like to thank All India Institute of Medical Science (AIIMS) Bhopal, Madhya Pradesh, India, for providing support during the study.
Declaration
I guarantee this is my original work, and I have the rights in the work. I am submitting the work for first publication in this journal and it is not being considered for publication elsewhere. It has not already been published elsewhere, and I have obtained and can supply all necessary permissions for the reproduction of any copyright works not owned by you.
Ethical approval
Ref No.-IHEC-LOP/2019/EF0111 by, Institutional Human Ethics Committee, All India Institute of Medical Sciences (AIIMS) Bhopal, Madhya Pradesh, India.
Conflict of Interest
None declared.
Funding
None.
References
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249.
- [CrossRef] [PubMed] [Google Scholar]
- Beyond mammography: new frontiers in breast cancer screening. Am J Med. 2013;126:472-479.
- [CrossRef] [PubMed] [Google Scholar]
- Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36:2105-2122.
- [CrossRef] [PubMed] [Google Scholar]
- Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5:5-23.
- [CrossRef] [PubMed] [Google Scholar]
- Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-172.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical management of the breast: breast conservation therapy and mastectomy. Surg Clin North Am. 2013;93:411-428.
- [CrossRef] [PubMed] [Google Scholar]
- Survival Study of Triple-Negative and Non-Triple-Negative Breast Cancer in a Brazilian Cohort Homero Gonçalves Maximiliano Ribeiro Guerra, Jane Rocha Duarte Cintra, Vívian Assis Fayer, Igor Vilela Brum, Maria Teresa Bustamante Teixeira. 2018. [Internet]. [cited 2021 Jul 26]. Accessed August 25, 2023 https://journals.sagepub.com/doi/full/10.1177/1179554918790563
- [Google Scholar]
- A perspective on the diagnostics, prognostics, and therapeutics of microRNAs of triple-negative breast cancer. Biophys Rev. 2019;11:227-234.
- [CrossRef] [PubMed] [Google Scholar]
- Role of micro-RNAs in breast cancer surgery. Br J Surg. 2018;105:e19-e30.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting miRNAs involved in cancer stem cell and EMT regulation: an emerging concept in overcoming drug resistance. Drug Resist Updat. 2010;13:109-118.
- [CrossRef] [PubMed] [Google Scholar]
- MicroRNA and drug resistance. Cancer Gene Ther. 2010;17:523-531.
- [CrossRef] [PubMed] [Google Scholar]
- MicroRNA-34 family: a potential tumor suppressor and therapeutic candidate in cancer. J Exp Clin Cancer Res. 2019;38:53.
- [CrossRef] [PubMed] [Google Scholar]
- miR-34a expression in human breast cancer is associated with drug resistance. Oncotarget. 2017;8:106270-106282.
- [CrossRef] [PubMed] [Google Scholar]
- Good guy or bad guy: the opposing roles of microRNA 125b in cancer. Cell Commun Signal. 2014;12:30.
- [CrossRef] [PubMed] [Google Scholar]
- The emerging role of microRNAs in breast cancer. J Oncol. 2020;2020:9160905.
- [CrossRef] [PubMed] [Google Scholar]
- MicroRNA-125b upregulation confers aromatase inhibitor resistance and is a novel marker of poor prognosis in breast cancer. Breast Cancer Res. 2015;17:13.
- [CrossRef] [PubMed] [Google Scholar]
- Role of miR-21 as an authentic oncogene in mediating drug resistance in breast cancer. Gene. 2020;738:144453. Doi: 10.1016/j.gene.2020.144453
- [CrossRef] [PubMed] [Google Scholar]
- microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer. 2019;19:738.
- [CrossRef] [PubMed] [Google Scholar]
- Role of miR-221/222 in tumor development and the underlying mechanism. J Oncol. 2019;2019:7252013.
- [CrossRef] [PubMed] [Google Scholar]
- The role of miR-200 family in the regulation of hallmarks of cancer. Front Oncol. 2022;12:965231. https://www.frontiersin.org/articles/10.3389/fonc.2022.965231
- [PubMed] [Google Scholar]
- miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest. 2014;124:5109-5128.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic and prognostic value of miR-200 family in breast cancer: A meta-analysis and systematic review. Cancer Epidemiol. 2022;77:102097.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer Staging System [Internet] American College of Surgeons [cited 2021 Jul 29]. Accessed August 25, 2023 http://www.facs.org/quality-programs/cancer/ajcc/cancer-staging
- [Google Scholar]
- Clinical Cancer Investigation Journal [Internet] [cited 2021 Jul 24]. Accessed August 25, 2023: https://www.ccijonline.org/article.asp?issn¼2278-0513;year¼2012;volume¼1;issue¼4;spage¼201;epage¼205;aulast¼Saha
- [CrossRef] [Google Scholar]
- Triple negative breast cancer: an Indian perspective. Breast Cancer (Dove Med Press). 2015;7:239-243.
- [CrossRef] [PubMed] [Google Scholar]
- TNBC vs non-TNBC: a retrospective review of differences in mean age, family history, smoking history, and stage at diagnosis. Clin Adv Hematol Oncol. 2014;12:377-381.
- [CrossRef] [Google Scholar]
- Epidemiology of breast cancer in Indianwomen. Asia Pac J Clin Oncol. 2017;13:289-295.
- [CrossRef] [PubMed] [Google Scholar]
- A study of triple negative breast cancer at a tertiary cancer care center in southern India. Ann Med Health Sci Res. 2014;4:933-937.
- [CrossRef] [PubMed] [Google Scholar]
- Triple-negative breast cancer risk in women is defined by the defect of estrogen signaling: preventive and therapeutic implications. OncoTargets Ther. 2014;7:147-164.
- [CrossRef] [PubMed] [Google Scholar]
- Family history of breast cancer in first-degree relatives and triple-negative breast cancer risk. Breast Cancer Res Treat. 2011;126:671-678.
- [CrossRef] [PubMed] [Google Scholar]
- Family history and riskof breast cancer: an analysis accounting for family structure. Breast Cancer Res Treat. 2017;165:193-200.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathological comparison of triple negative breast cancers with non-triple negative breast cancers in a hospital in North India. Niger J Clin Pract. 2015;18:381-386.
- [CrossRef] [PubMed] [Google Scholar]
- Association between breast cancer laterality and tumor location, United States, 1994-1998. Cancer Causes Control. 2004;15:637-645.
- [CrossRef] [PubMed] [Google Scholar]
- Family history of breast and ovarian cancer and triple negative subtype in Hispanic/Latina women. Springerplus. 2014;3:727.
- [CrossRef] [PubMed] [Google Scholar]
- Breast cancer care in India: the current scenario and the challenges for the future. Breast Care (Basel). 2008;3:21-27.
- [CrossRef] [PubMed] [Google Scholar]
- Metabolic syndrome and triple-negative breast cancer: a new paradigm. Int J Breast Cancer. 2012;2012:809291.
- [CrossRef] [PubMed] [Google Scholar]
- DecreasedserummiR-181ais a potential new tool for breast cancer screening. Int J Mol Med. 2012;30:680-686.
- [CrossRef] [PubMed] [Google Scholar]
- The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res Treat. 2010;121:479-483.
- [CrossRef] [PubMed] [Google Scholar]
- Weight gain prior to diagnosis and survival from breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1803-1811.
- [CrossRef] [PubMed] [Google Scholar]
- Triple-negative breast cancer and its association with obesity. Mol Clin Oncol. 2017;7:935-942.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of basal-like and luminal breast cancers among black women in the AMBER Consortium. Cancer Epidemiol Biomarkers Prev. 2021;30:71-79. Doi: 10.1158/1055-9965.EPI-20-0556
- [CrossRef] [PubMed] [Google Scholar]
- Breast size, handedness and breast cancer risk. Eur J Cancer. 1991;27:131-135.
- [CrossRef] [PubMed] [Google Scholar]
- Metastatic and triple-negative breast cancer: challenges and treatment options. Drug Deliv Transl Res. 2018;8:1483-1507.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding patterns of brain metastasis in triple-negative breast cancer and exploring potential therapeutic targets. OncoTargets Ther. 2021;14:589-607.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating microRNAs in plasma as early detection markers for breast cancer. Int J Cancer. 2013;132:1602-1612.
- [CrossRef] [PubMed] [Google Scholar]
- MicroRNA-125 in immunity and cancer. Cancer Lett. 2019;454:134-145.
- [CrossRef] [PubMed] [Google Scholar]
- MiR-125b regulates the proliferation and metastasis of triple negative breast cancer cells via the Wnt/ß-catenin pathway and EMT. Biosci Biotechnol Biochem. 2019;83:1062-1071.
- [CrossRef] [PubMed] [Google Scholar]
- High expression of miR-21 in triple-negative breast cancers was correlated with a poor prognosis and promoted tumor cell in vitro proliferation. Med Oncol. 2014;31:57.
- [CrossRef] [PubMed] [Google Scholar]
- miR221/222 in cancer: their role in tumor progression and response to therapy. Curr Mol Med. 2012;12:27-33.
- [CrossRef] [PubMed] [Google Scholar]
- MicroRNA-200c inhibits the metastasis of triple-negative breast cancer by targeting ZEB2, an epithelial-mesenchymal transition regulator. Ann Clin Lab Sci. 2020;50:519-527.
- [Google Scholar]
- Low expression of circulating MicroRNA-34c is associated with poor prognosis in triple-negative breast cancer. Yonsei Med J. 2017;58:697-702.
- [CrossRef] [PubMed] [Google Scholar]
- miRNA-21 promotes proliferation and invasion of triple-negative breast cancer cells through targeting PTEN. Am J Transl Res. 2017;9:953-961.
- [Google Scholar]
- Triple-negative breast cancer: role of specific chemotherapy agents. Cancer J. 2010;16:53-61.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of resistance of chemotherapy in earlystage triple negative breast cancer (TNBC) Breast. 2017;34:S27-S30.
- [CrossRef] [PubMed] [Google Scholar]
- Predictive biomarkers for triple negative breast cancer treated with platinum-based chemotherapy. Cancer Biol Ther. 2017;18:369-378.
- [CrossRef] [PubMed] [Google Scholar]
- Potential of miR-21 to predict incomplete response to chemoradiotherapy in rectal adenocarcinoma. Front Oncol. 2020;10:577653.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating MiR-125b as a marker predicting chemoresistance in breast cancer. PLoS One. 2012;7:e34210.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of triple-negative breast cancer in India: systematic review and metaanalysis. J Glob Oncol. 2016;2:412-421.
- [CrossRef] [PubMed] [Google Scholar]
- Alarming burden of triple-negative breast cancer in India. Clin Breast Cancer. 2018;18:e393-e399.
- [CrossRef] [PubMed] [Google Scholar]
- Triplenegative breast cancer: current perspective on the evolving therapeutic landscape. Int J Womens Health. 2019;11:431-437.
- [CrossRef] [PubMed] [Google Scholar]
- Triple negative breast cancer: a review of present and future diagnostic modalities. Medicina (Kaunas). 2021;57:62.
- [CrossRef] [PubMed] [Google Scholar]





