Translate this page into:
A comparative study to evaluate liquid dish washing soap as an alternative to xylene and alcohol in deparaffinization and hematoxylin and eosin staining
Address for correspondence: Dr. Pinki Pandey, E-mail: pnkdxt@yahoo.co.in
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Our study presents a new deparaffinizing and hematoxylin and eosin (H and E) staining method that involves the use of easily available, nontoxic and eco-friendly liquid diluted dish washing soap (DWS) by completely eliminating expensive and hazardous xylene and alcohol from deparaffinizing and rehydration prior to staining, staining and from dehydration prior to mounting. The aim was to evaluate and compare the quality of liquid DWS treated xylene and alcohol free (XAF) sections with that of the conventional H and E sections.
Materials and Methods:
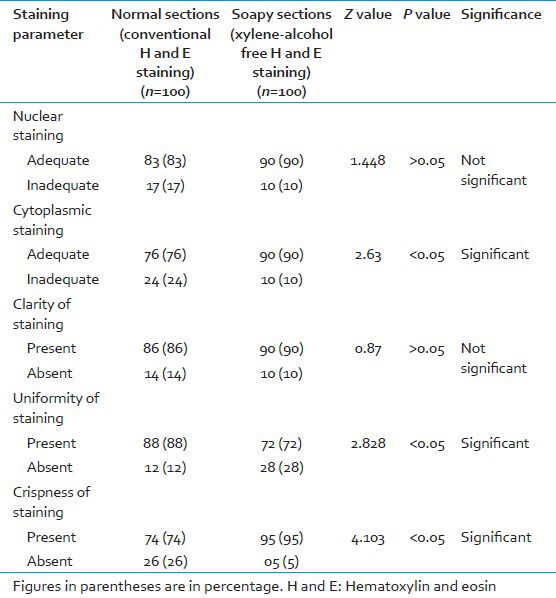
A total of 100 paraffin embedded tissue blocks from different tissues were included. From each tissue block, one section was stained with conventional H and E (normal sections) and the other with XAF H and E (soapy sections) staining method. Slides were scored using five parameters: Nuclear, cytoplasmic, clarity, uniformity, and crispness of staining. Z-test was used for statistical analysis.
Results:
Soapy sections scored better for cytoplasmic (90%) and crisp staining (95%) with a statistically significant difference. Whereas for uniformity of staining, normal sections (88%) scored over soapy sections (72%) (Z = 2.82, P < 0.05). For nuclear (90%) and clarity of staining (90%) total scored favored soapy sections, but the difference was not statistically significant. About 84% normal sections stained adequately for diagnosis when compared with 86% in soapy sections (Z = 0.396, P > 0.05).
Conclusion:
Liquid DWS is a safe and efficient alternative to xylene and alcohol in deparaffinization and routine H and E staining procedure. We are documenting this project that can be used as a model for other histology laboratories.
Keywords
Alcohol
deparaffinization
eosin
hematoxylin
liquid dish washing soap
xylene-free staining
INTRODUCTION
Medical laboratory jobs in general and histology tasks in particular are not risks-free activities because of the wide range of chemical, mechanical, biologic, and environmental hazards the histotechinician is exposed to, all of which can pose immediate or long-term health consequences. Basic aim in any field of life sciences is to utilize eco-friendly cheap nontoxic, less biohazardous chemicals. Any attempt to reduce health hazards in histology laboratories deserves to be tried.
As the common saying goes “simple is best” and so it is that hematoxylin and eosin (H and E) has stood the test of time. Even today, 150 years after its introduction, it is still the most frequently used staining method in anatomical pathology worldwide.[1] H and E staining is remarkably robust and works well with a variety of fixatives. It is a primary contrast method in medical diagnosis of biopsy specimens and used to discriminate between a broad range of cytoplasmic, nuclear and extracellular matrix features.[2] Xylene and graded alcohols are the components in the gold standard conventional H and E staining procedure, apart from H and E. However, its (i.e., xylene and alcohol) demerits are cost containment, toxicity, and polluted working environment.[3]
Xylene, a mixture of three aromatic hydrocarbon isomers related to benzene, widely used in industries and medical technology as a solvent. It is a colorless, sweet smelling liquid or gas occurring naturally in petroleum, coal, and wood tar. It is also worth noting that xylene is present in many household solvents, air fresheners, stainless steel cleaners, floor polishers, and gasoline. It is used in histopathology laboratories for tissue processing, deparaffinizing the tissue sections, coverslipping, cleaning tissue processors, and recycling. Its high solvency factor allows maximum displacement of alcohol and renders the tissue transparent, enhancing paraffin infiltration. In staining procedures, its excellent dewaxing and cleaning capabilities contribute to brilliant stained slides.[3456] Exposure to alcohol occurs during tissue processing and deparaffinizing the tissue sections before staining.
The hazards of xylene are well-documented, making it a potential occupational hazard for the histopathological technicians. Exposure to xylene can occur via inhalation, ingestion, eyes and skin. Toxic effects of xylene include acute neurotoxicity, cardiac and kidney injuries, hepatotoxicity, fatal blood dyscrasias, skin erythema, drying, scaling, secondary infection and also have a carcinogenic effect.[45]
The National Institute of Occupational Safety and Health recommended exposure limits for xylene at 100 ppm as a time-weighted average for up to a 10 h work shift and a 40 h work week and 200 ppm for 10 min as a short term limit.[78]
After the hazardous effects of xylene became indisputable in the 1970s, many potential substitutes became available in order to make a xylene-free environment in laboratories, such as limonene reagents, aliphatic hydrocarbons, aromatic hydrocarbons, olive oil, vegetable oils, and mineral oil substitute. However, these chemicals were used to substitute xylene as a clearing agent during routine processing, while the exposure and handling of xylene is maximum during deparaffinization of the tissue sections.[459]
Falkeholm et al., for the first time experimented to use liquid dish washing soap (DWS) to dewax the tissue sections by eliminating both xylene and alcohol from H and E staining procedures.[3]
The liquid DWS is a mixture of surfactants composed of sodium laureth sulfate, cocamidopropyl betaine, sodium dodecyl benzenesulfonate and nonionic surfactants. These anionic surfactants, lowers the surface tension of water and commonly used in detergent soaps and shampoos.[10] Detergent dish soap made with ingredients of the highest quality, high performance and strong power easily removes grease from kitchen utensils, glassware, pans, etc., and has neutral pH 7 that does not mistreat hands, leaving them soft and with a pleasant aroma.[11]
Different research works published in the past, have also used liquid DWS instead of xylene and alcohol, and concluded that staining the tissues with xylene-methanol free method was at par with the conventional staining procedure.[5689] Thus, the present study was designed to substitute hazardous compounds like xylene and alcohol, used in deparaffinization and staining, with cheap easily available liquid DWS having no toxicity and inflammability without impairing the morphology, staining characteristics and diagnostic values of the tissue sections. Thus, we are documenting the project in a pilot study that can be used as a model for other histology laboratories. Our aims and objectives were:
-
To test the hypothesis that xylene and alcohol free (XAF) sections deparaffinized with diluted DWS are better than or at par with the conventional H and E sections
-
To evaluate and compare the quality of XAF H and E sections with that of the conventional H and E sections`
-
To assess the potential of 1.7% liquid DWS as a xylene substitute for histopathology.
MATERIALS AND METHODS
The present prospective study was carried out in the Department of Pathology for a period of 6 months.
Reagents
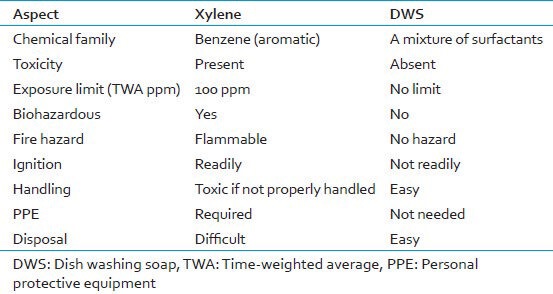
1.7% liquid DWS was used as an alternative substitute for xylene and alcohol. Liquid DWS forms a part of the household products which are used daily. Their concentration in these products is already well monitored by the manufacturing companies. We used Vim liquid dish-washing soap (Hindustan Unilever Limited) in our study. It is easily available, cheap, safe, nontoxic and eco-friendly. Moreover, we are diluting only 25 ml of the liquid DWS in 1500 ml of distilled water. Thus, there are skimpy chances of this product being toxic to the laboratory personnel. The physicochemical aspects of xylene and DWS are elucidated in Table 1.
Harri's hematoxylin was used for conventional staining method, whereas Mayer's hematoxylin was used to stain the nuclear component in XAF staining method in our study. Mayer's hematoxylin was prepared by completely dissolving aluminum potassium sulfate (alum) in distilled water. Then adding hematoxylin, sodium iodate and acetic acid to it and bring it to boil and cool.[12] This solution is alcohol free and gives clear and sharp nucleus staining.
Tissue samples
A total of 100 surgically resected specimens from different tissues were obtained. The study group included tissues such as epithelium, connective tissue, glands, bone, cartilage, and muscle. The specimens were fixed in 4% neutral buffered formaldehyde and routinely processed as per conventional method. For the pilot study, a total of 100 paraffin embedded tissue blocks were included.
Histological procedure
Two sections of 4 μm each were cut from 100 paraffin embedded tissue blocks, thus a total of 200 sections were examined. Eliminating xylene and alcohol during deparaffinization and staining are validated in our article following the same methodology published elsewhere.[36] Deparaffinization is a key point in the XAF method and was done using diluted 1.7% liquid DWS at 90°C. The histological procedures for the two groups were carried out in parallel by the same histotechnicians. One section was stained with conventional H and E method and the other with XAF H and E staining method. Staining of the conventional sections was preceded by rehydration, followed by dehydration in alcohol and clearing with xylene before mounting, whereas these steps were unnecessary for the XAF sections. Staining follows immediately after deparaffinization. Oven drying the XAF sections at 60°C prior to cover slipping are sufficient as depicted in Table 2.

Assessment of stained sections
All the 200 stained section were coded as normal sections (conventional H and E stained sections) and soapy sections (XAF H and E stained sections). A randomized mix of 200 sections gave 100 matched pairs. Every section was scored and analyzed without the prior knowledge of staining method employed. The study was thus single blind and also prevented the observer bias. An individual section was objectively analyzed using a scoring system used by Ankle et al.[6] to enable comparison between the two staining methods.
H and Estained sections were graded based on the following five parameters:
-
Nuclear staining (adequate = score 1, inadequate = score 0)
-
Cytoplasmic staining (adequate = score 1, inadequate = score 0)
-
Clarity of staining (present = score 1, absent = score 0)
-
Uniformity of staining (present = score 1, absent = score 0)
-
Crispness of staining (present = score 1, absent = score 0).
A score of 0-1 was given to each of these parameters and the slides were graded by adding up the scores. A score of ≤2 was graded as inadequate for diagnosis, and the slides with score 3-5 were assigned as adequate for diagnosis. “Z” test for proportions was used to compare the two staining methods, P < 0.05 was considered as significant.
RESULTS
As regards the scores for the parameters cytoplasmic staining (90%) and crisp staining (95%), a statistically significant difference in favor of XAF H and E staining method was observed. As far as uniformity of staining is concerned, conventional H and E staining method (88%) scored over XAF H and E staining method (72%) with a statistically significant difference (Z = 2.82, P < 0.05). For the rest of the parameters-nuclear staining and clarity of staining, the total score favored XAF H and E staining method but the difference was not statistically significant [Table 3 and Figure 1].
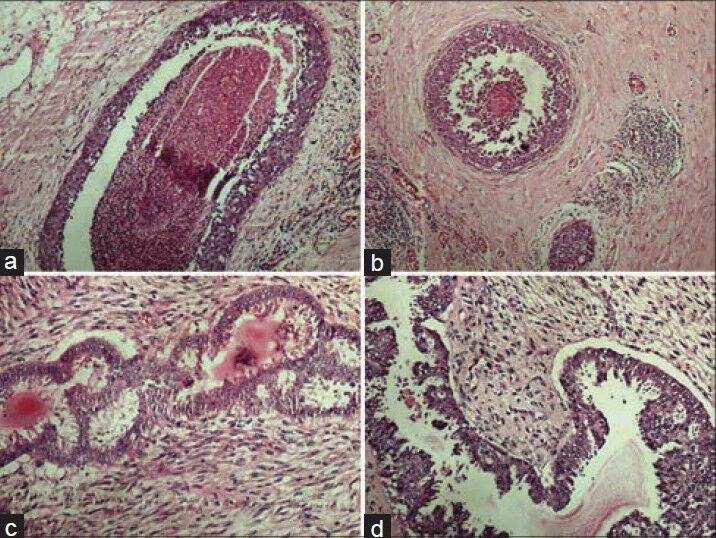
- Photomicrographs showing crisp staining in breast tissue (a) DCIS with conventional (H and E, ×100), (b) DCIS with xylene-alcohol free (XAF) (H and E, ×100), (c) Phyllodes tumor with conventional (H and E, ×200), (b) Phyllodes tumor with XAF (H and E, ×200)
On comparing the adequacy of diagnosis of stained sections obtained by the two staining methods on the basis of scores obtained, it was apparent [Table 4] that more number of diagnostically adequate (86) and less number of diagnostically inadequate (14) sections were obtained by XAF H and E staining method, but this difference was not statistically significant (Z = 0.396, P > 0.05).

DISCUSSION
Xylene traditionally has been employed as a clearing, dewaxing, and mounting agent for histology.[5913] It is well-documented as an environmental hazard and highly toxic to humans. Being repeatedly or excessively exposed to xylene can do harm to the nervous system, skin, liver, kidney, and lung tissues. In addition, xylene has many shortcomings, such as being highly flammable and volatile, much lower boiling point (137-143°C), a flash point (25°C) and an ignition point (25°C).[13]
For a number of years, xylene has been widely used in the histology laboratories in spite of its toxicity to personnel and the danger it poses to the environment.[14] The historical use of xylene in the histology laboratory is an example of futile substitution. Due to new regulations from Occupational Safety and Health Administrations several xylene substitutes have been commercially developed in recent years. However, most of the commercially available xylene substitutes are less effective, more expensive, and not that much less hazardous than xylene itself.[514]
Any technique that minimizes the use of xylene by using non-biohazardous substitutes, reduces staining time with unequivocal cell morphology will be indispensable for diagnostic reasons as well as for maintaining a healthy laboratory environment, thereby minimizing the risk to the laboratory personnel. Thus, our study presents the results of a new deparaffinizing and H and E staining method that involves the use of easily available, nontoxic and eco-friendly liquid diluted DWS by completely eliminating expensive and hazardous xylene and alcohol from deparaffinizing and rehydration prior to staining, staining and from dehydration prior to mounting, so as to device an optimal staining technique which is easily available, less toxic, time-saving, and cost-effective.
The nucleus reflects the reproductive potential of the cell. The size and staining intensity (chromasia) of the nucleus is a critical factor in the evaluation of the cell. Correct hematoxylin staining shows crisp staining of the nuclear chromatin, demonstrated well delineated nuclear membranes and sharply stained condensed chromatin against an unstained nucleoplasm.
When the results were compared for nuclear staining, it was found that 90% of the XAF H and E sections showed adequate nuclear staining when compared with 83% of conventional H and E sections [Table 3 and Figure 2]. The difference was not statistically significant (Z = 1.448, P > 0.05), suggesting that there was no difference in the two staining methods.
- Photomicrographs showing adequacy and clarity of nuclear and cytoplasmic staining. (a) Conventional (H and E, ×200), (b) Xylenealcohol free (H and E, ×200)
The staining strategy for application of hematoxylin can be grouped into two major paradigms; progressive and regressive. In progressive staining, the reaction is stopped once the desired staining intensity is achieved. There is no differentiation required (thereby saving time) unlike with the regressive hematoxylin stains like Harri's and Delafield's hematoxylin.[15] Mayer's hematoxylin was used to stain the nuclear component in XAF staining method in our study. Nuclear staining with Mayer's hematoxylin was achieved in only 11 min unlike 20 min required for Harri's hematoxylin used for conventional H and E sections. This saved time and simplified the staining procedure. Similar to previous studies the degree of crispness of nuclear staining with Mayer's hematoxylin was found to be nearly equivalent to Harri's hematoxylin.[36]
In contrast to our study, Ramulu et al. used Harri's hematoxylin in xylene ethanol free staining method. As it is a regressive stain, it had to be differentiated with 1% acid alcohol. If differentiation is omitted or incomplete following regressive staining, residual hematoxylin visually obscures fine chromatin detail and can prevent the uptake of eosin entirely due to binding of aluminum hematin in the cytoplasm.[16] They noted that in spite of the differentiation step, few sections still showed bluish tinge, which was rectified by increasing the eosin staining time for 30 s more.[8]
Cytoplasmic staining was downgraded significantly in conventional H and E stained sections as compared with the XAF H and E sections. 90% of XAF sections showed adequate cytoplasmic staining pattern as compared with 76% of the conventional H and E sections (Z = 2.63, P < 0.05) [Table 3].
Commercially available water soluble 1% eosin Y (Selkrom Company, Mumbai, India) was used with the XAF staining. Out of 100 sections, 10 showed inadequate cytoplasmic stain in XAF sections. These sections showed bluish tinge of cytoplasm and seemed deteriorated. This finding was similar to that of other studies.[68] Eosin is an acidic dye and provides its maximum uptake to the basic cytoplasmic proteins when used at pH 5 to 5.3.[17] It will rinse out in any alkaline solution. The pH of eosin was found to be adequate. Lithium carbonate was the source of alkalinity. The other source of alkalinity was the tap water (pH 7.02) wash used before and after the eosin staining step. This increase of the alkalinity had probably resulted in eosin being washed out, resulting in a light staining. Lithium carbonate was eliminated because pH of tap water was found to be adequate for bluing, which alleviated this problem.
Akin to the results observed by some of the previous workers.[68] the clarity of staining pattern was found to be better by XAF H and E staining (90%) as compared to conventional H and E staining (86%). The difference was not statistically significant (Z = 0.87, P > 0.05), suggesting that XAF H and E staining method is equivalent/at par with conventional H and E.
As far as uniformity of staining was concerned, a statistically significant difference in favor of conventional H and E staining was observed. 88% of conventional H and E sections showed uniform staining pattern as compared with 72% of the XAF sections (Z = 2.828, P < 0.05) [Table 3]. Patchy staining and out-of-focus areas in XAF sections downgraded the uniform staining pattern in 28 out of 100 sections. Similar findings were observed in the experimental study done by Ankle et al. as well as Ramulu et al.[68] This error could be due to tear or fold in sections, thick sections, moisture on coverslip, dirty microscopic lenses or, improper deparaffinization.[18]
A noteworthy finding observed in our study was XAF staining method is highly temperature sensitive. The diluted 1.7% liquid DWS I, II and the distilled water I and II had to be strictly maintained at 90°C. Even the slightest increase in temperature resulted in washing away of the tissue sections from the slide and on the other hand, if the temperature was less than 90°C or even if the sections were kept in 1.7% liquid DWS I, II and distilled water I and II for lesser time than recommended, the sections were not completely deparaffinized leaving areas of residual wax, resulted in out-of-focus areas. An additional, 1.7% liquid DWS III was added in the staining protocol for 30 s at 90°C, to rectify this error.
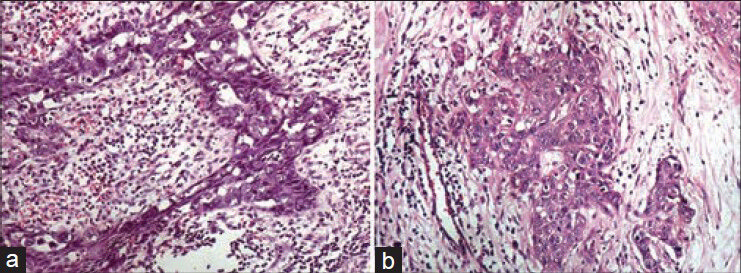
As regards the scores for crispness of staining was concerned, a statistically significant difference in favor of XAF H and E staining was observed (Z = 4.103, P < 0.05) [Figures 3 and 4]. 95% of the XAF sections revealed a crisp staining pattern when compared with 74% of the conventional H and E sections contrasting to the results observed by some of the previous workers.[8] Our observation is analogous to that of Ankle et al.,[6] where XAF sections showed a significant upgradation in crispness as compared with conventional H and E. The combination of 1.7% liquid DWS along with water soluble Mayer's H and E brings near ideal degree of crispness.
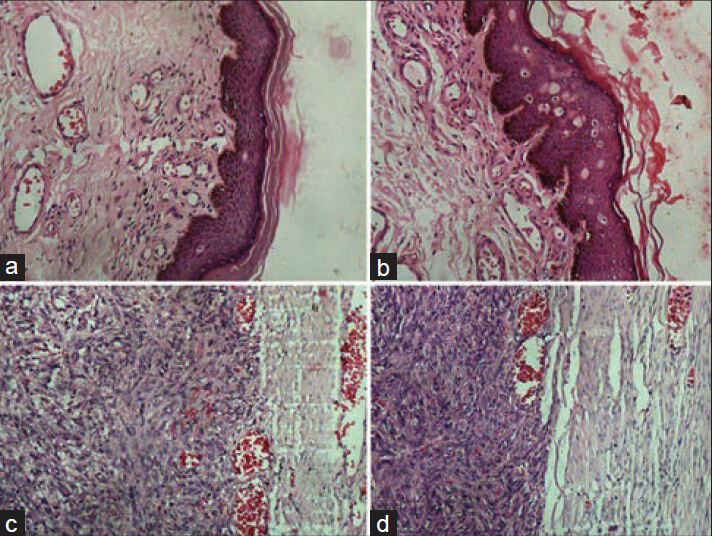
- Photomicrographs showing crispness of staining in epithelial and connective tissues. (a) Epithelial tissue stained with conventional (H and E, ×200), (b) Epithelial tissue stained with xylene-alcohol free (XAF) (H and E, ×200), (c) Connective tissue stained with conventional (H and E, ×200), (b) Connective tissue stained with XAF (H and E, ×200)
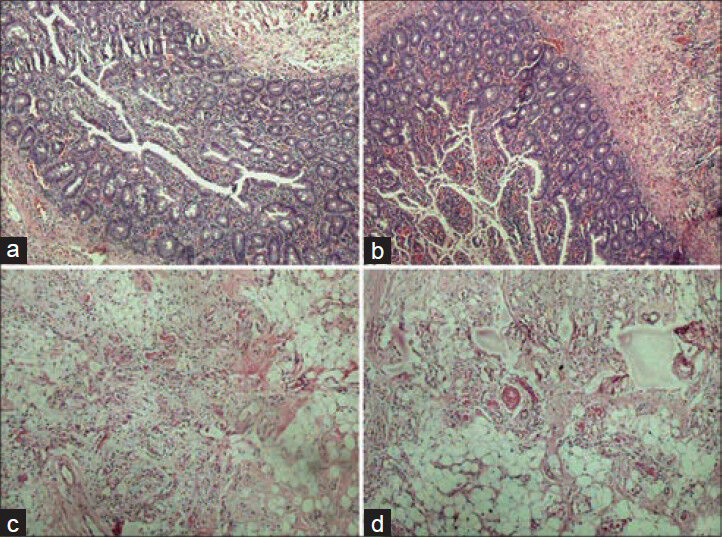
- Photomicrographs showing crisp staining in glandular tissue and adipose tissue, (a) Glandular tissue with conventional (H and E, ×100), (b) Glandular tissue with xylene-alcohol free (XAF) (H and E, ×100), (c) Adipose tissue with conventional (H and E, ×100), (b) Adipose tissue with XAF (H and E, ×100)
When we compared the adequacy of diagnosis of stained sections obtained by the two staining methods on the basis of scores obtained, it was evident that 86% of the XAF H and E stained sections were found to be adequate for diagnosis as compared with 84% of the conventional H and E (Z = 0.396, P > 0.05). This is at variance to the results observed by some of the previous workers.[8] XAF sections were ranked as good as or better than their conventional counterparts in 74% of the comparisons and poorer in 26% in the study done by Falkeholm et al.[3] In an attempt to completely eliminate xylene from the staining process, Buesa and Peshkov demonstrated that the use of 1.7% dishwasher soap aqueous solution at 90°C to dewax sections before staining and oven drying prior to coverslipping will eliminate xylene from the staining process.[59]
The need for minimal turnaround time has encouraged innovations in staining procedures that require lesser staining time with unequivocal cell morphology. The turnaround time for the entire deparaffinization and staining protocol of XAF method was dramatically reduced. Deparaffinization with liquid DWS was achieved in only 4 min unlike 20 min required in conventional H and E (using xylene and alcohol). The whole staining procedure was completed in 30-35 min using liquid DWS whereas 70-75 min was needed for the corresponding conventional H and E method.
The histology technicians who processed, deparaffinized and stained the slides were also interviewed regarding the use of 1.7% DWS. They offered very helpful feedback on advantages and disadvantages of both xylene and the substitute 1.7% DWS. The health benefits to workers using 1.7% DWS in place of xylene are first characterized by the lack of noxious odors in the laboratory. Many technicians are irritated by the noxious fumes in the histopathology laboratory, even at low levels. DWS has no bad odors and does not seem to elicit irritation responses in laboratory workers. The technicians’ comments are depicted in Table 5. Furthermore, after being stored for more than a 6 months, the slides were well preserved.

However, our study had a small sample size and was for shorter duration and so it is highly recommended to conduct further studies with large sample size covering varied tissues. Prudence is advised to be observed while interpreting our conclusions as many tissues were not covered in this pilot study.
Thus, using liquid DWS treated XAF H and E sections for many specimens successfully; we strongly believe that XAF H and E sections are qualitatively at par with conventional H and E sections for routine diagnostic work. It produces a quality staining with a good maintenance of cell morphology and structure and a clear definition of the cytoplasm and the nucleus. Liquid DWS is a safe and efficient alternative to xylene and alcohol and may probably replace them without losing valuable diagnostic information's. Finally, substituting xylene with liquid DWS is highly desirable, as it has an additional advantage of being nonhazardous, nontoxic, eco-friendly, and time and cost-effective. We are documenting this project that can be used as a model for other histology laboratories.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Q Imaging Camera Application Notes. H and Estain tissue documentation. Available from: http://www.qimaging.com/support/pdfs/he_technote.pdf
- [Google Scholar]
- Xylene-free method for histological preparation: A multicentre evaluation. Lab Invest. 2001;81:1213-21.
- [Google Scholar]
- Xylene: An overview of its health hazards and preventive measures. J Oral Maxillofac Pathol. 2010;14:1-5.
- [Google Scholar]
- A study to evaluate the efficacy of xylene-free hematoxylin and eosin staining procedure as compared to the conventional hematoxylin and eosin staining: An experimental study. J Oral Maxillofac Pathol. 2011;15:161-7.
- [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH) Criteria for a recommended standard: Occupational exposure to xylene. 1975. Available from: http://www.cdc.gov/niosh/75-168.html
- [Google Scholar]
- Liquid dish washing soap: An excellent substitute for xylene and alcohol in hematoxylin and eosin staining procedure. J Orofac Sci. 2012;4:37-42.
- [Google Scholar]
- Complete elimination of xylene in practice of a histology laboratory. Arkh Patol. 2011;73:54-60.
- [Google Scholar]
- Available from: http://www.en.wikipedia.org/wiki/Detergent
- Technical Information Guide. Available from: http://www.technogreen.us/wp-content/uploads/2011/09/3_Dish-Soap2.pdf
- [Google Scholar]
- Mayer's Hematoxylin Protocol. Available from: http://www.ihcworld.com/_protocols/counterstain_solutions/mayer-hematoxylin.htm
- [Google Scholar]
- A novel xylene substitute for histotechnology and histochemistry. Biotech Histochem. 2010;85:231-40.
- [Google Scholar]
- Mineral oil: The best xylene substitute for tissue processing yet? J Histotechnol. 2000;23:143-9.
- [Google Scholar]
- The haematoxylins and eosin. In: Bancroft JD, Stevens A, eds. Theory and Practice of Histological Techniques (4th ed). New York: Churchill-Livingstone; 1996. p. :99-112.
- [Google Scholar]
- H and E staining: Oversights and insights. Available from: http://www.dako.com/08066_12may10_webchapter 13.pdf
- [Google Scholar]
- Problems in histopathological technique. Available from: http://www.ihcworld.com/royellis/problems/problem25.htm
- [Google Scholar]
- Histotechnique and staining troubleshooting. 2009. On Atlas of Mohs and Frozen Section Cutaneous Pathology. New York: Springer Link; Available from: http://www.books.google.co.in/books
- [Google Scholar]





